Physical And Chemical Properties Of Metals в Selftution
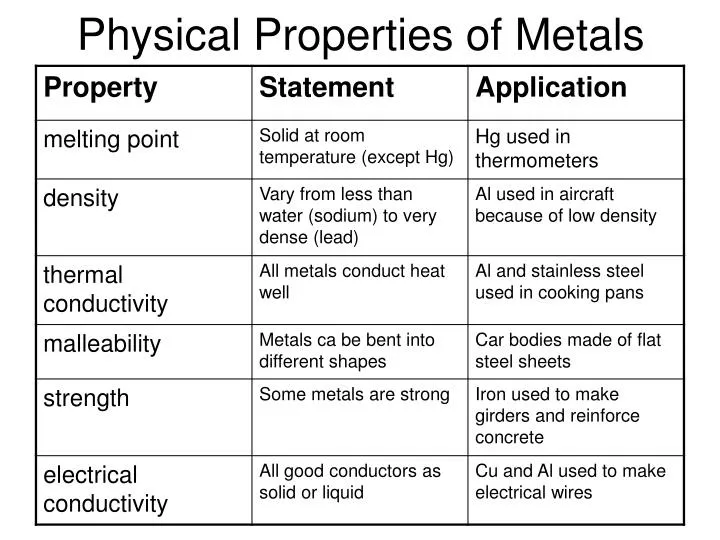
Ppt Physical Properties Of Metals Powerpoint Presentation Id 5519685 Some basic physical and chemical properties of metals that differentiate them from non metals and metalloids are as follows: physical properties of metals: usually, metals are hard, opaque, possess luster, and conduct heat and electricity. they are malleable and ductile, can withstand longitudinal pull, and produce a resonant sound when struck. The difference in properties of metals, nonmetals, and metalloids. state: metals and metalloids are generally solid at room temperature, whereas nonmetals exist as liquids, gases, or brittle solids. metalloids are brittle solids that crumble to powder when struck. luster: metals are lustrous i.e. show brightness, whereas nonmetals are non lustrous.

Physical And Chemical Properties Of Metals в Selftution Summary. a physical property is a characteristic of a substance that can be observed or measured without changing the identity of the substance. physical properties include color, density, hardness, and melting and boiling points. a chemical property describes the ability of a substance to undergo a specific chemical change. A metal in chemistry is defined as an element that can easily form positive ions called cations and tends to make metallic bonds. the metals are distinguished by their chemical and physical properties such as malleability, ductility, ionization and bonding properties etc. properties of metals . examples of metals are gold, aluminium, iron and. Density. electric conductivity, thermal conductivity. metallic characteristics variation going down the group and along a period (left to right) melting and boiling points. variation of chemical reactivity of metals with oxygen, water, acids and more chemicals. reactivity with cold water, hot water and steam. reactivity with acids and bases. The chemical elements can be broadly divided into metals, metalloids, and nonmetals according to their shared physical and chemical properties.all elemental metals have a shiny appearance (at least when freshly polished); are good conductors of heat and electricity; form alloys with other metallic elements; and have at least one basic oxide.

Comments are closed.