Polyprotic Acids Bases Titration Curves Youtube

Polyprotic Acids Bases Titration Curves Youtube Titrating a polyprotic acid with a strong base produces a ph curve with as many equivalence points as there are acidic protons on the acid. the pkₐ values fo. This lesson follows what is present in the sample flask at each point in the titration of a polyprotic weak acid with a strong base, emphasizing what mathema.
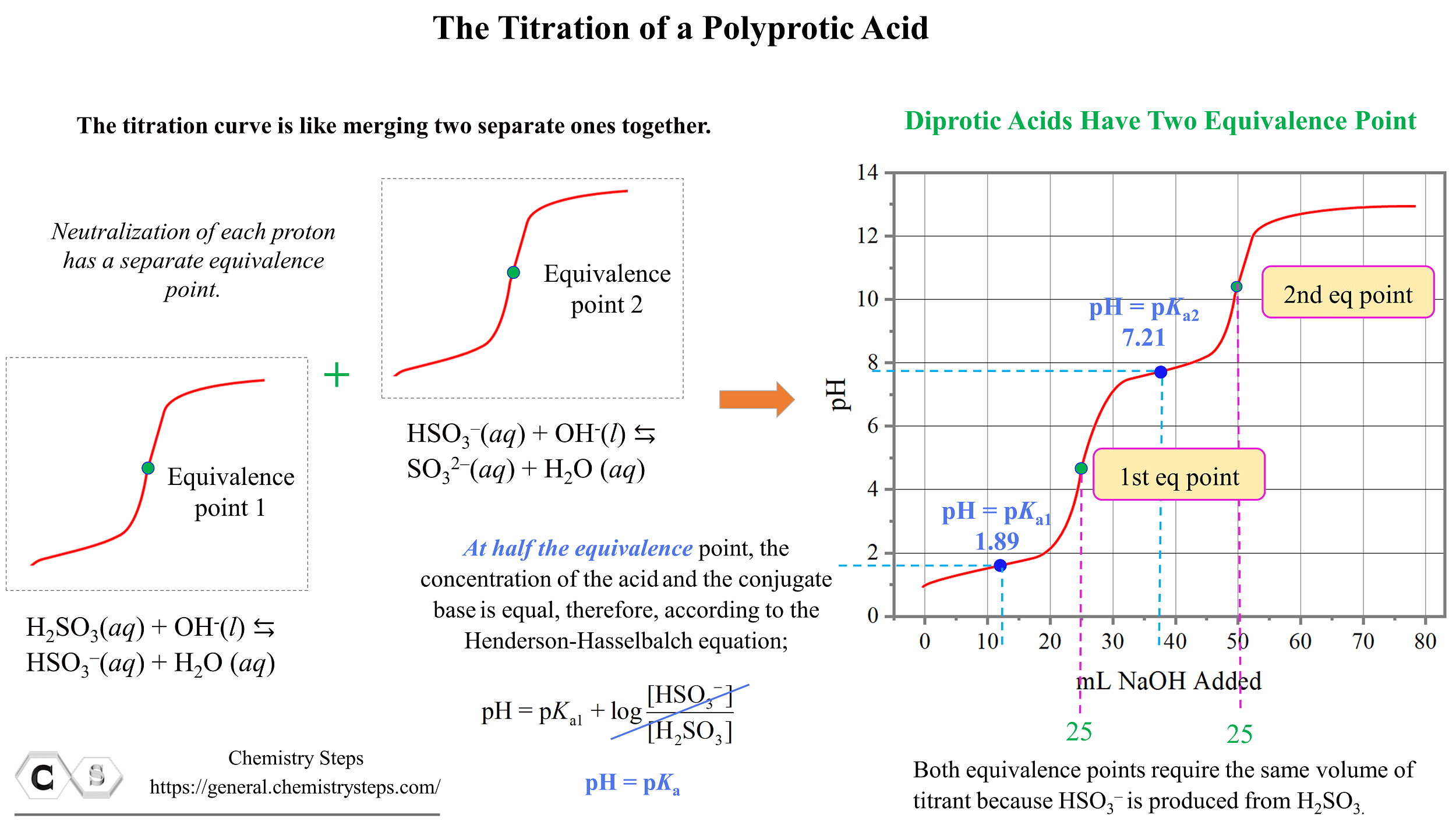
Titration Of A Polyprotic Acids Chemistry Steps This chemistry video tutorial provides a basic introduction to acid base titrations. it shows you how to calculate the unknown concentration of an acid solu. D30.2 titration of polyprotic acids and bases. when a polyprotic acid is titrated, there are usually multiple equivalence points. for example, when h 2 so 3 is titrated with naoh, there are two equivalence points corresponding to the two acidic protons from the h 2 so 3 molecule. there are also as many midpoints as there are equivalence points. The weak polyprotic acid (analyte) is in green and is titrated with teh strong base (the titrant) in red. (cc by; heather yee via libretexts) when an acid is titrated, there is an equivalence, or stoichiometric, point, which is when the moles of the strong base added equal of the moles of weak acid present. however, when a weak polyprotic acid. In the titration of a polyprotic acid like sulfurous acid with a strong base such as sodium hydroxide, the base first neutralizes the initial ionizable proton, forming an intermediate species (e.g., hydrogen sulfite ions). this step's titration curve resembles that of a weak monoprotic acid, with a half equivalence point where the ph equals the.

Polyprotic Acid Base Part 4 Titrations Indicators Youtube The weak polyprotic acid (analyte) is in green and is titrated with teh strong base (the titrant) in red. (cc by; heather yee via libretexts) when an acid is titrated, there is an equivalence, or stoichiometric, point, which is when the moles of the strong base added equal of the moles of weak acid present. however, when a weak polyprotic acid. In the titration of a polyprotic acid like sulfurous acid with a strong base such as sodium hydroxide, the base first neutralizes the initial ionizable proton, forming an intermediate species (e.g., hydrogen sulfite ions). this step's titration curve resembles that of a weak monoprotic acid, with a half equivalence point where the ph equals the. D39.4 titration of polyprotic acids and bases. when a polyprotic acid is titrated, there are usually multiple equivalence points. for example, when h 2 so 3 is titrated with naoh, there are two equivalence points corresponding to the two acidic protons from the h 2 so 3 molecule. there are also as many midpoints as there are equivalence points. Polyprotic acids. polyprotic acids are acids that can lose several protons per molecule. they can be further categorized into diprotic acids and triprotic acids, those which can donate two and three protons, respectively. the best way to demonstrate polyprotic acids and bases is with a titration curve.

Regions Of Titration Curve And Polyprotic Acids Youtube D39.4 titration of polyprotic acids and bases. when a polyprotic acid is titrated, there are usually multiple equivalence points. for example, when h 2 so 3 is titrated with naoh, there are two equivalence points corresponding to the two acidic protons from the h 2 so 3 molecule. there are also as many midpoints as there are equivalence points. Polyprotic acids. polyprotic acids are acids that can lose several protons per molecule. they can be further categorized into diprotic acids and triprotic acids, those which can donate two and three protons, respectively. the best way to demonstrate polyprotic acids and bases is with a titration curve.

Acid Base Equilibria Polyprotic Acids Titration Youtube

Comments are closed.