Potentiometric Acid Base Titrations

Potentiometric Acid Base Titrations Youtube In a potentiometric titration you record the ph as you add titrant, and if the analyte is a weak acid or base you can determine its \(k a\) or \(k b\). figure \(\pageindex{1}\): generic apparatus for an acid base titration. you take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know concentration. In analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. it is a useful means of characterizing an acid. no indicator is used; instead the electric potential is measured across the analyte, typically an electrolyte solution. to do this, two electrodes are used, an indicator electrode (the.
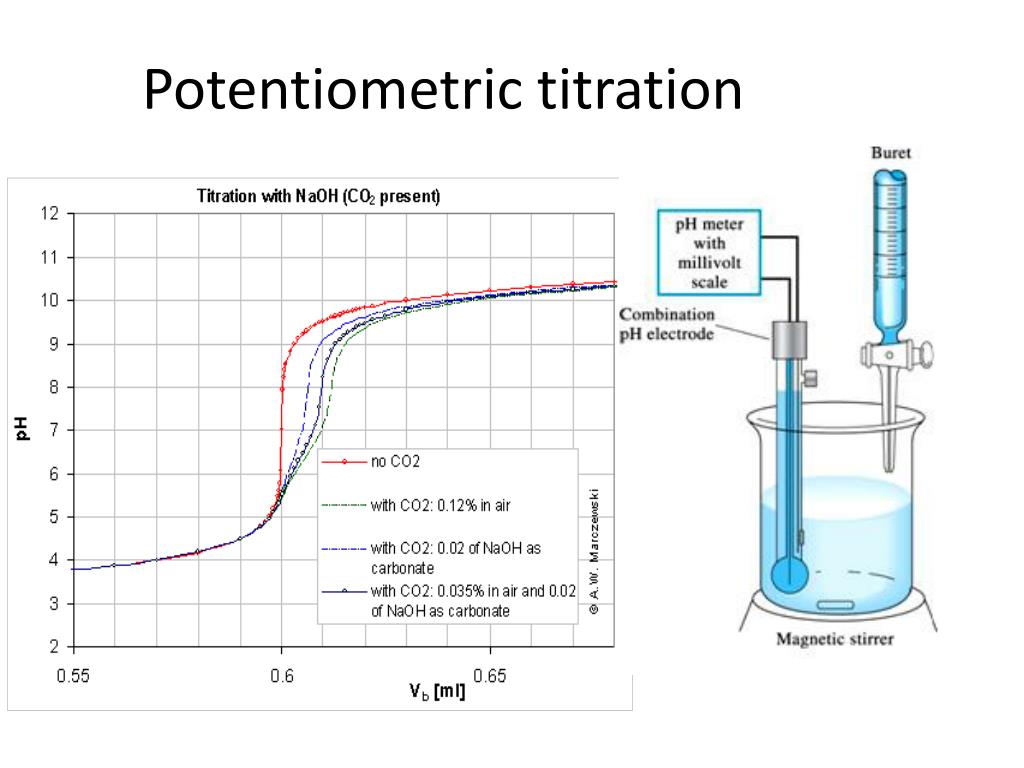
Ppt Potentiometry Powerpoint Presentation Free Download Id 5410570 Acid base titration: this type of potentiometric titration is used to determine the concentration of a given acid base by neutralizing it exactly using a standard solution of base acid whose concentration is known. redox titration: this type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. an example of. Record the final volume (vf) to 0.01 ml. note 1: add only 2~3 drops of indicator to the solution to avoid titration error, for the indicator is also a weak acid. note 2: prior to titration, the titration volume of naoh solution can be estimated from the number of moles of khp using a stoichiometric calculation. 5. Acid–base, complexation, and precipitation potentiometric titrations are usually monitored with an ion selective electrode selective for the analyte, although an electrode selective for the titrant or a reaction product also can be used. a redox electrode, such as a pt wire, and a reference electrode are used for potentiometric redox. A potentiometric titration is similar to the acid base titrations you have performed; however, the means of determining the concentration of a species is dependent upon an oxidation reduction reaction rather than a neutralization reaction.

Comments are closed.