Pressure Temperature P T Diagram And Equation

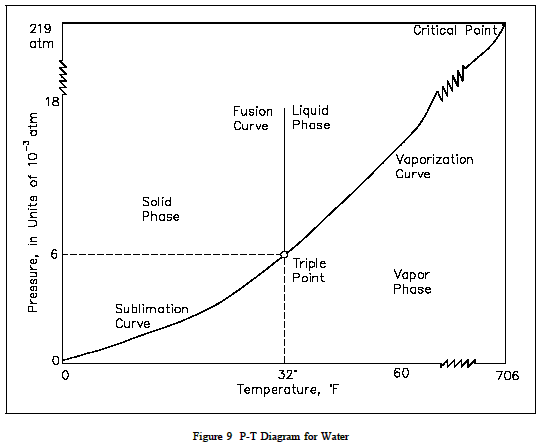
Pressure Temperature Diagram P T Diagram Fundamentals Of Fluid F From the p t diagram, figure 2.3.e1, co 2 at 100 bar, 275 k is in the liquid phase. the isobaric process is shown as the horizontal, yellow line with a constant pressure of 100 bar, see figure 2.3.e1. at approximately 220 k, the isobaric process line meets the fusion line, and the liquid co 2 starts to change to solid co 2. Pressure temperature (p t) diagram and equation. a p t diagram for water type of property diagram. figure 10 is the p t diagram for pure water. a p t diagram can be constructed for any pure substance. a p t diagram is different from a p t diagram in one particularly important way. there are regions on a p n diagram in which two phases exist.
Pressure Temperature P T Diagram Thermodynamics Where the ∝ symbol is read “is proportional to.” a plot of v versus 1 p is thus a straight line whose slope is equal to the constant in equation 6.2.1 and equation 6.2.3. dividing both sides of equation 6.2.1 by v instead of p gives a similar relationship between p and 1 v. the numerical value of the constant depends on the amount of gas. Solution. from the p t diagram, the liquid phase can only exist when the pressure is great than the triple point pressure. from appendix f, table f1, co 2 has a triple point pressure of 517 kpa and a triple point temperature of 216.55 k (−56.60 °c), respectively; therefore, the lowest pressure for liquid co 2 to exist is 517 kpa. This point represents the end of the liquid–gas equilibrium curve. this point is also semantically important to define different regions of the phase diagram, as in figure 12.3.1. a gas whose pressure and temperature are below the critical point is called a vapor. a gas whose temperature is above the critical one and the pressure is below its. Guillaume amontons was the first to empirically establish the relationship between the pressure and the temperature of a gas (~1700), and joseph louis gay lussac determined the relationship more precisely (~1800). because of this, the p t relationship for gases is known as either amontons’s law or gay lussac’s law.

Comments are closed.