Process Validation
Quality Pharma Gxp This guidance outlines the general principles and approaches for process validation of drugs and biologics, based on a product lifecycle concept and existing fda guidance. it covers the stages of process design, qualification, and verification, and provides recommendations and references for manufacturers. Learn about the analysis of data gathered throughout the design and manufacturing of a product to confirm that the process can reliably output products of a determined standard. find out the three stages of process validation: process design, process qualification, and continued process verification.

Process Validation Explained Learn about the stages, types, and examples of process validation, the guidance of fda and ghtf for validating pharmaceutical manufacturing processes, and how to effectively establish documented evidence of process validation. the web page also covers the concepts of process design, qualification, verification, and revalidation, and provides a process validation study plan template. Learn what process validation is and how to apply a lifecycle approach to ensure product quality and consistency. this presentation covers the fda guidance, the three stages of process validation, and the key activities and considerations for each stage. Learn how to validate your manufacturing process to ensure consistent product quality and compliance with regulatory requirements. this guide covers the stages, types, and approaches of process validation, as well as the tools and documents needed for a successful validation plan. Learn about the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological products. this guidance applies to active pharmaceutical ingredients (apis or drug substances) and products.
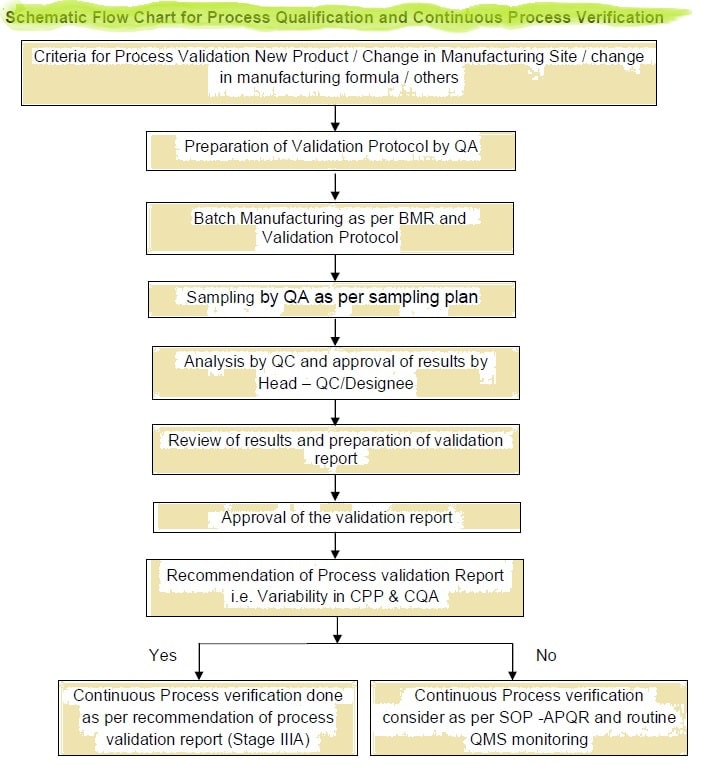
Process Validation Pv Verification Of Drug Product Guidelines Sops Learn how to validate your manufacturing process to ensure consistent product quality and compliance with regulatory requirements. this guide covers the stages, types, and approaches of process validation, as well as the tools and documents needed for a successful validation plan. Learn about the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological products. this guidance applies to active pharmaceutical ingredients (apis or drug substances) and products. Learn what process validation is, why it is important, and how to implement it in the manufacturing industry. this guide covers the key steps, regulatory requirements, and challenges of process validation. Learn what process validation is, when and how to perform it, and what regulatory requirements apply. find out the difference between validation, verification, and pq, iq, and oq, and see examples of process validation in different contexts.
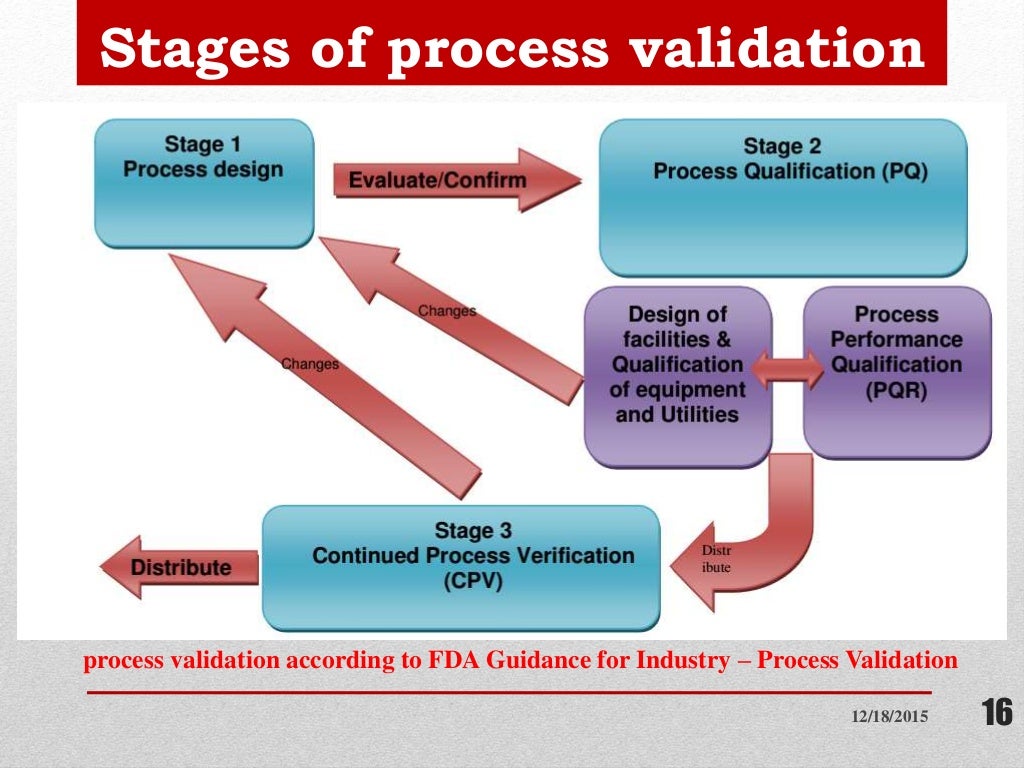
Process Validation Learn what process validation is, why it is important, and how to implement it in the manufacturing industry. this guide covers the key steps, regulatory requirements, and challenges of process validation. Learn what process validation is, when and how to perform it, and what regulatory requirements apply. find out the difference between validation, verification, and pq, iq, and oq, and see examples of process validation in different contexts.

Comments are closed.