Properties Of Metals

Physical Properties Of Metals Full List With Examples Teachoo Learn about 12 physical and chemical properties of metals, such as hardness, density, conductivity, and alkaline nature. see examples of common metals and their uses in daily life. Learn the basic properties of metals, nonmetals, and metalloids based on their placement in the periodic table. metals are electropositive, lustrous, malleable, ductile, and good conductors of heat and electricity.

Properties Of Metals The chemical elements can be broadly divided into metals, metalloids, and nonmetals according to their shared physical and chemical properties. all elemental metals have a shiny appearance (at least when freshly polished); are good conductors of heat and electricity; form alloys with other metallic elements; and have at least one basic oxide. Learn about metals, their physical and chemical properties, and how they are used in various applications. find out the complete list of metals with atomic numbers, symbols and elements, and their reactions with water and acids. Learn about the characteristics and examples of metals, nonmetals, and metalloids, the three types of elements in the periodic table. metals are shiny, malleable, and conduct heat and electricity, while nonmetals are dull, brittle, and form negative ions. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. approximately three quarters of all known chemical elements are metals. the most abundant varieties in the earth’s crust are aluminum, iron, calcium, sodium, potassium, and magnesium.

Physical Properties Of Metals And Alloys Illustration Stock Image Learn about the characteristics and examples of metals, nonmetals, and metalloids, the three types of elements in the periodic table. metals are shiny, malleable, and conduct heat and electricity, while nonmetals are dull, brittle, and form negative ions. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability, ductility, and high reflectivity of light. approximately three quarters of all known chemical elements are metals. the most abundant varieties in the earth’s crust are aluminum, iron, calcium, sodium, potassium, and magnesium. A metal is a material that conducts electricity and heat well, and has a lustrous appearance. learn about the different types, forms, structures and properties of metals, and see examples of common metals and their uses. Three properties of metals are: luster: metals are shiny when cut, scratched, or polished. malleability: metals are strong but malleable, which means that they can be easily bent or shaped. for centuries, smiths have been able to shape metal objects by heating metal and pounding it with a hammer.
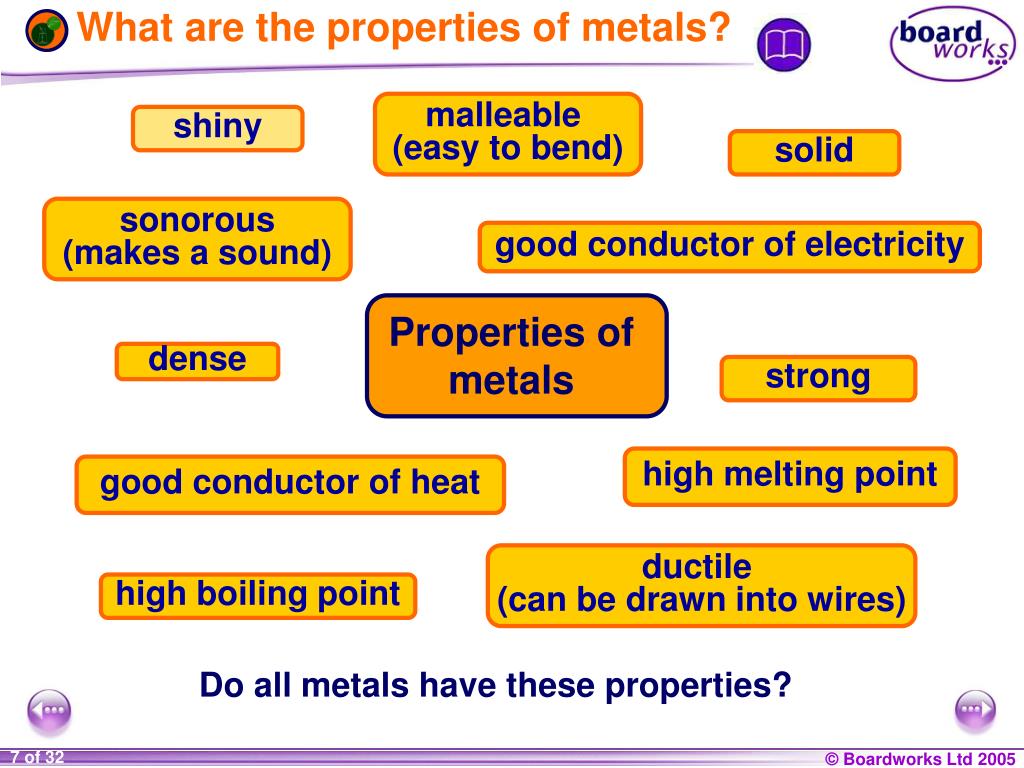
Ppt Ks3 Chemistry Powerpoint Presentation Free Download Id 3804641 A metal is a material that conducts electricity and heat well, and has a lustrous appearance. learn about the different types, forms, structures and properties of metals, and see examples of common metals and their uses. Three properties of metals are: luster: metals are shiny when cut, scratched, or polished. malleability: metals are strong but malleable, which means that they can be easily bent or shaped. for centuries, smiths have been able to shape metal objects by heating metal and pounding it with a hammer.
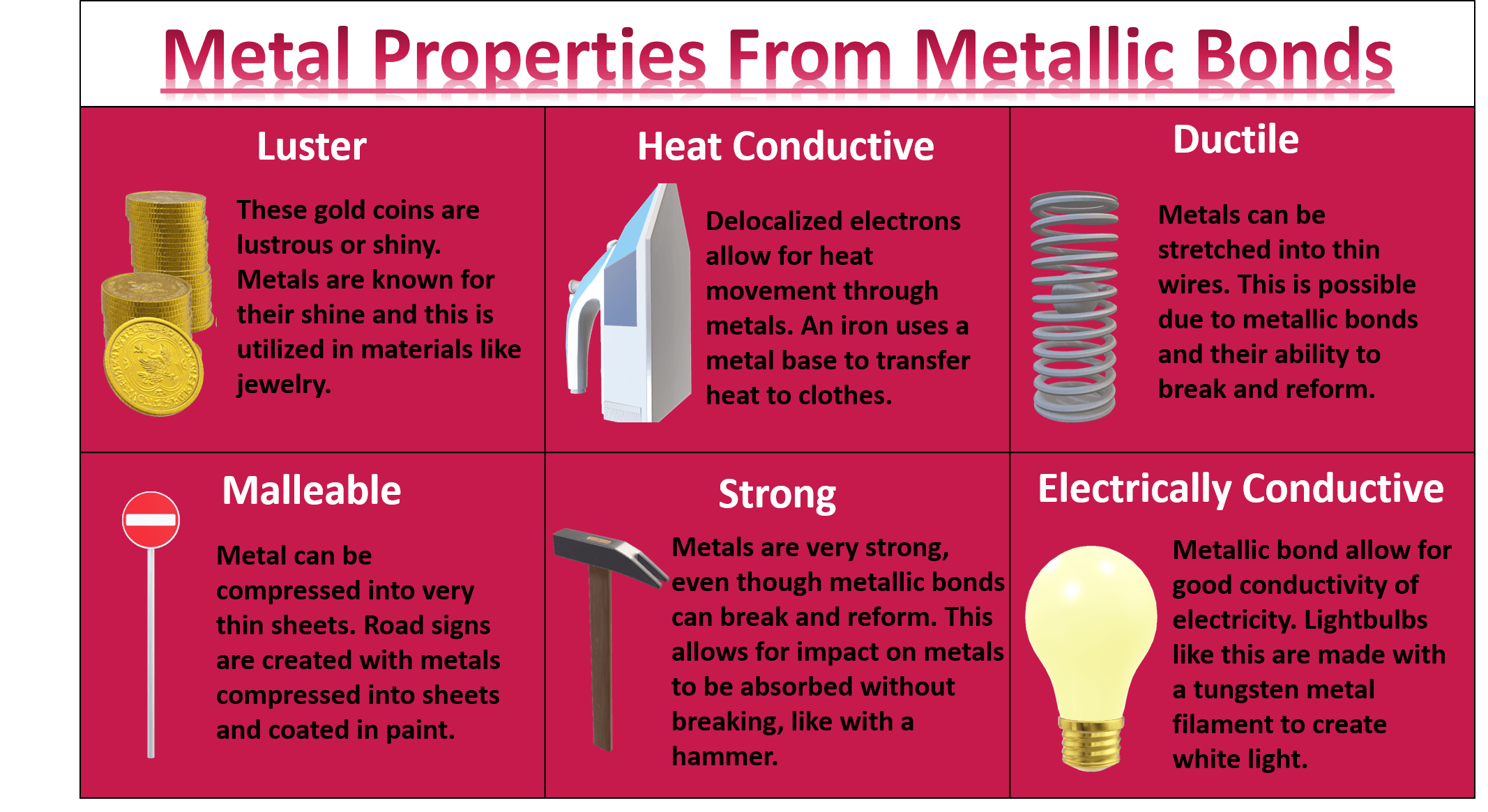
Why Are Metals Shiny At George Day Blog

Comments are closed.