Pvt Phase Diagram
Pvt Phase Diagram With Pressure Versus Volume And Isotherms Tn Thin The pvt surface above represents a substance which contracts upon freezing. most substances do so, the notable exception being water which expands upon freezing. a considerable amount of information about the phases of matter can be illustrated with the pvt surface. the solid, liquid and gas (vapor) phases can be represented by regions on the. From the p t diagram, figure 2.3.e1, co 2 at 100 bar, 275 k is in the liquid phase. the isobaric process is shown as the horizontal, yellow line with a constant pressure of 100 bar, see figure 2.3.e1. at approximately 220 k, the isobaric process line meets the fusion line, and the liquid co 2 starts to change to solid co 2.
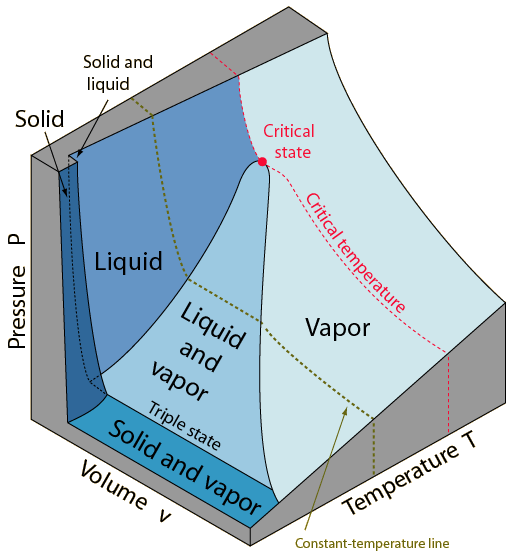
Phase Diagrams Of Pure Substances I love this 3d diagram!you are able to see pressure, volume and temperaturejust choose your 2 d vision and you will get any of the diagrams exposed before:p. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Explore thousands of free applications across science, mathematics, engineering, technology, business, art, finance, social sciences, and more. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist.

Comments are closed.