Qualitative Analysis Of Group Iii A Cations Chemistry Not Mysteryођ

Qualitative Analysis Of Group Iii A Cations Chemistry Not Now let's continue from the group ii filtrate and discuss the steps we have to follow to separate iii (a) cations. qualitative analysis of group iii (a) cations. step 1: precipitation of iii (a) cations as hydroxide to the filtrate, add few drops of nitric acid hno3 to convert ferrous fe2 into ferric fe3 . Part b: analysis and identification of group iii cations in an unknown sample. obtain a test tube which contains a mixture of group iii cations. record the id code of the sample on your report form. transfer 2.0 ml of the above mixture into a 30 ml beaker and repeat the procedure used in part a.
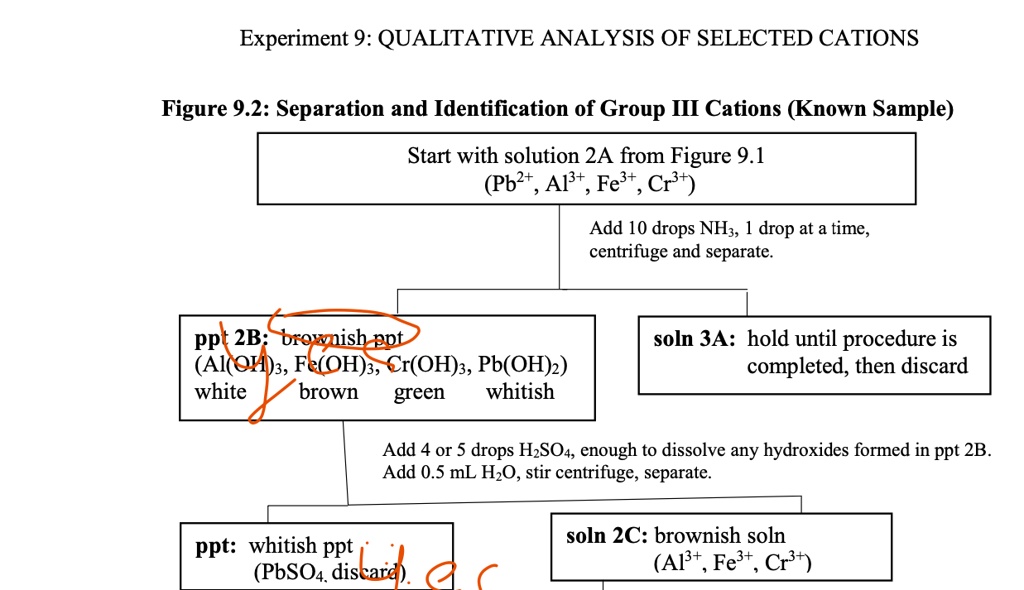
Solved Experiment 9 Qualitative Analysis Of Selected Cations Figure 9 Step 1: precipitation of iii (b) cations as sulphide. to the filtrate, add 2 3ml of 2m ammonia nh3 solution and heat, now pass hydrogen sulphide h2s gas under pressure for 0.5 to 1 min. you will get the precipitate of iii (b) cations which may contain cos, nis, mns and zns. h2s gas should be passed in hot solution for complete precipitation. 5.1: separation of group iii cations after insoluble chlorides and acid insoluble sulfides of group i and group ii has been removed, the medium is made alkaline at ph ~9 where group iii cations precipitate out as hydroxides, i.e., iron(ii) hydroxide, iron (iii) hydroxide, chromium(iii) hydroxide, and nickel(ii) hydroxides. Group 1: insoluble chlorides. most metal chloride salts are soluble in water; only ag , pb2 , and hg2 2 form chlorides that precipitate from water. thus the first step in a qualitative analysis is to add about 6 m hcl, thereby causing agcl, pbcl 2, and or hg 2cl 2 to precipitate. if no precipitate forms, then these cations are not present. Step 1: preparation: if you are working on the analysis of only group iii ions, prepare a known solution by mixing 5 6 drops each of 0.1 m solutions of fe 3, al 3, cr 3, and ni 2 in a 30 ml beaker. also obtain an unknown to analyze at the same time for the presence of group iii cations and use about 20 24 drops in your analysis.

Comments are closed.