Serial Dilution Formula Calculator Method Uses Examples
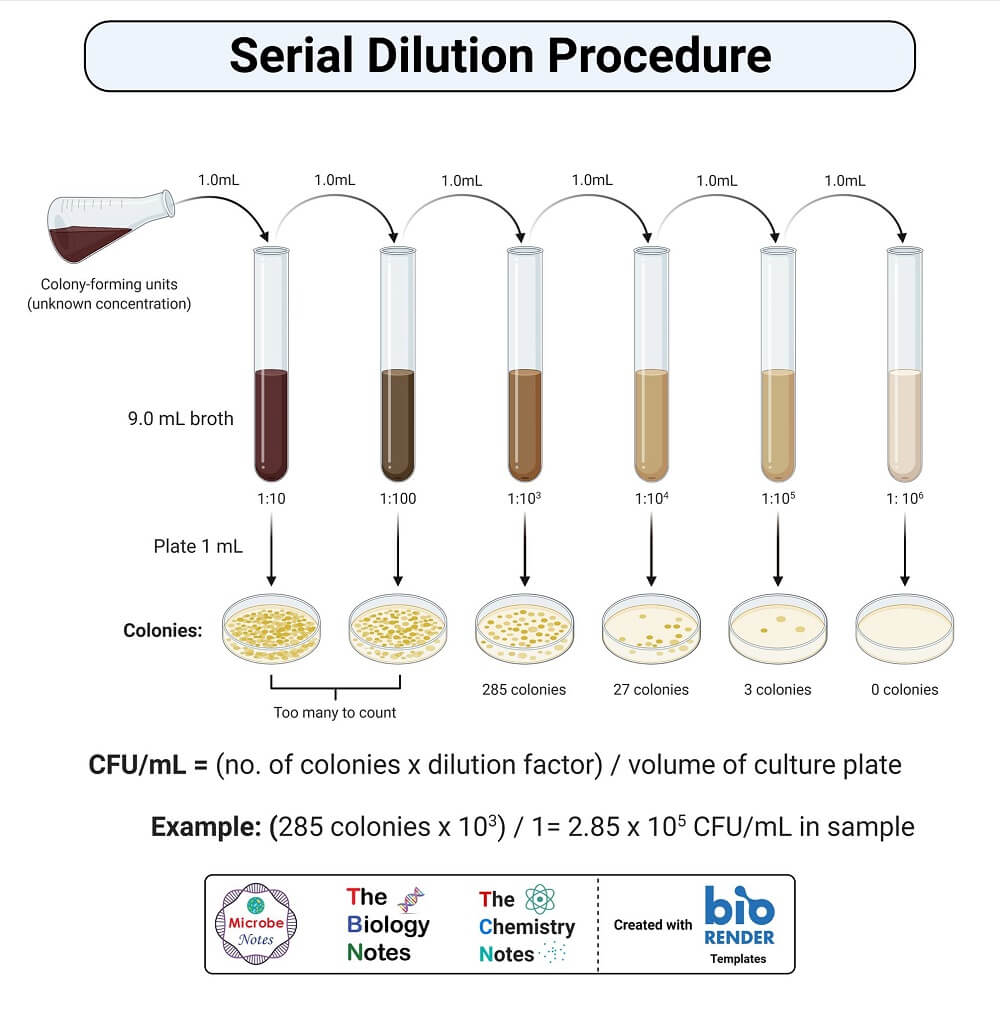
Serial Dilution Formula Calculator Method Uses Examples Serial dilution formula and calculations. serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 % saline. then, a small measured volume of each dilution is used to make a series of pour or spread plates. Serial dilutions have many uses that are mainly related to chemistry and biology. it may be useful to you if we elucidate some of the terms in this serial dilution calculator: method – you have two choices, dilution factor and concentration range. dilution factor allows you to input the dilution factor that separates one solution and the next.

Serial Dilution Formula Calculator Method Uses Examples The serial dilution method was first described in 1883 by german scientist and physician robert koch when he published his work on infectious disease causing agents,1 and is now a standard technique in today's laboratories. this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial dilution, as well as highlighting the. Serial dilution is a method of converting a dense solution into a more useable concentration by performing a series of repeated dilutions. serial dilution, in layman’s words, is the process of diluting a solution with a dilution factor one step at a time. serial dilution is commonly used in biology to reduce the concentration of cells in a. Total volume = (volume of sample volume of diluent), i.e., 1 ml sugar solution 9 ml water = 10 ml. dilution factor = total volume sample volume = 10 1= 10. this dilution can be expressed by various terms like. the dilution factor is 10 (dilution factor is the reciprocal of the dilution) it was a 10 fold dilution. Plug your dilution factor into the equation: d t = 10 x 10 x 10 x 10 = 10,000. the final dilution factor of the fourth tube in your serial dilution is 1:10,000. the concentration of your substance is now 10,000 times less than the original undiluted solution. 2. determine the concentration of the solution following dilution.

Comments are closed.