Sig Fig Rules Significant Figures
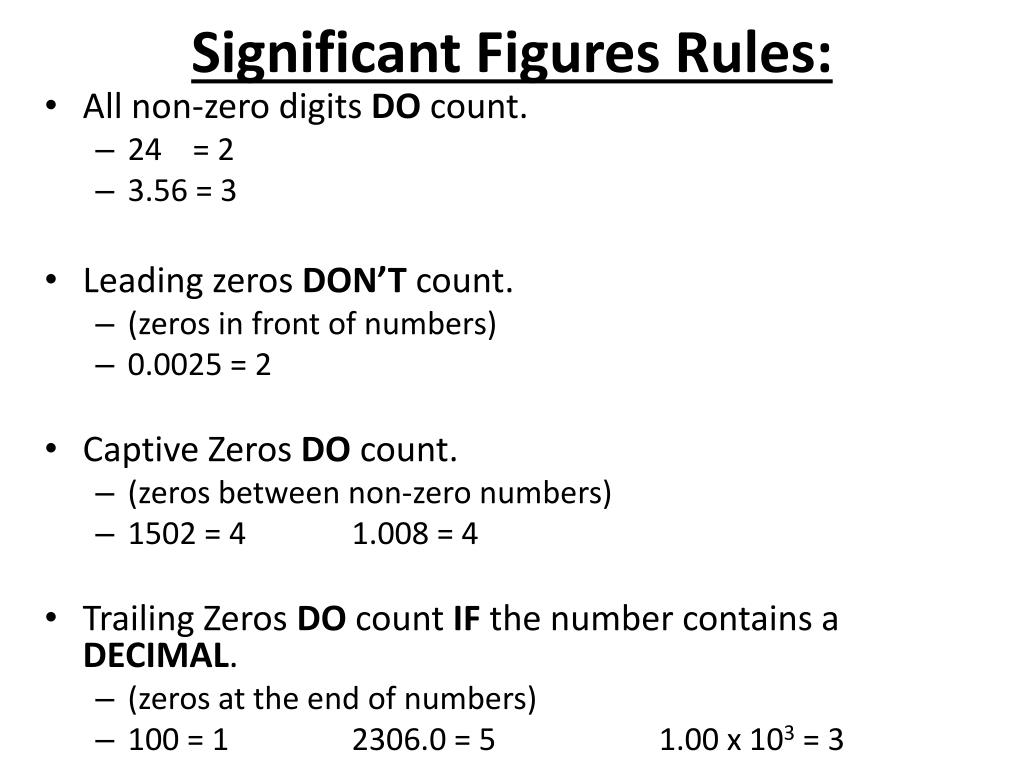
Significant Figures Chemistry Notes Rule 2 any zero contained between two non zero numbers is significant. if a zero forms part of a string of digits and falls between two non zero digits then it must be counted as a significant figure. examples: 1.05 has three significant figures (1, 0, 5); the zero is enclosed by non zero digits and should be counted. Solution: there are four significant figures in 67.30. final or trailing zeros are significant. 3. add these numbers by applying the significant figures rules: 42, 7.8, 6.50, 12. solution: 42 7.8 6.50 12 = 68.3. for the given numbers, the least number of significant digits on the right of the decimal point is 1.

Ppt Significant Figures Sig Fig Rules Powerpoint Presentation Free Our significant figures calculator works in two modes – it performs arithmetic operations on multiple numbers (for example, 4.18 2.33) or simply rounds a number to your desired number of sig figs. following the rules noted above, we can calculate sig figs by hand or by using the significant figures counter. The rules for determining the number of significant figures are as follows: all nonzero digits are significant. for example, the value 211.8 has four significant figures. all zeros that are found between nonzero digits are significant. thus, the number 20,007, with three 0s between the 2 and 7, has a total of five significant figures. Here are the fundamental sig fig rules: non zero digits are always significant. for example, in the measurement 3.14, all three digits are significant. zeros between non zero digits are significant. in the measurement 50.3, both the 5 and the 3 are significant. leading zeros (zeros to the left of the first non zero digit) are not significant. Rules for significant figures. all non zero digits are significant. 198745 contains six significant digits. all zeros that occur between any two non zero digits are significant. for example, 108.0097 contains seven significant digits. all zeros that are on the right of a decimal point and also to the left of a non zero digit is never significant.
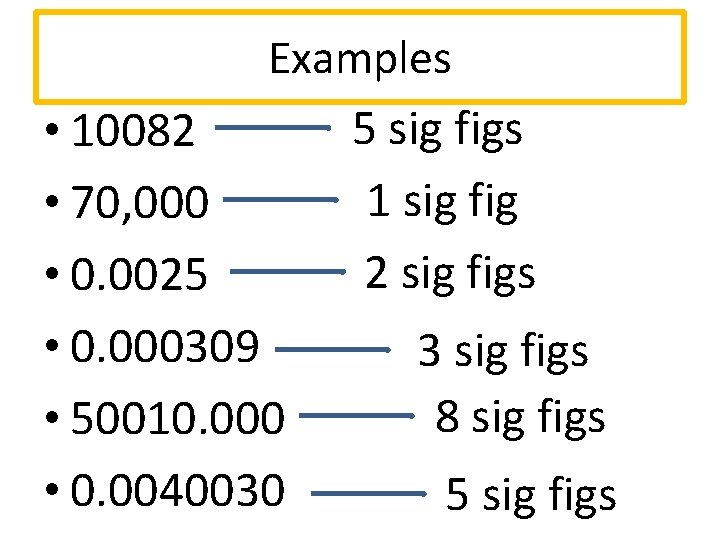
Significant Figures Sig Figs Sig Figs Scientists Use Here are the fundamental sig fig rules: non zero digits are always significant. for example, in the measurement 3.14, all three digits are significant. zeros between non zero digits are significant. in the measurement 50.3, both the 5 and the 3 are significant. leading zeros (zeros to the left of the first non zero digit) are not significant. Rules for significant figures. all non zero digits are significant. 198745 contains six significant digits. all zeros that occur between any two non zero digits are significant. for example, 108.0097 contains seven significant digits. all zeros that are on the right of a decimal point and also to the left of a non zero digit is never significant. 30.00 has 4 significant figures (3, 0, 0 and 0) and 2 decimals. 0.0025 has 2 significant figures (2 and 5) and 4 decimals. sig fig calculator operators. you can use the following operators and functions with our calculator: addition ( ), subtraction ( ), division ( or ÷ ) and multiplication ( * or × ). plus exponent ( ^ ). Explicitly state the number of significant figures (the abbreviation s.f. is sometimes used): for example "20 000 to 2 s.f." or "20 000 (2 sf)". state the expected variability (precision) explicitly with a plus–minus sign, as in 20 000 ± 1%. this also allows specifying a range of precision in between powers of ten.
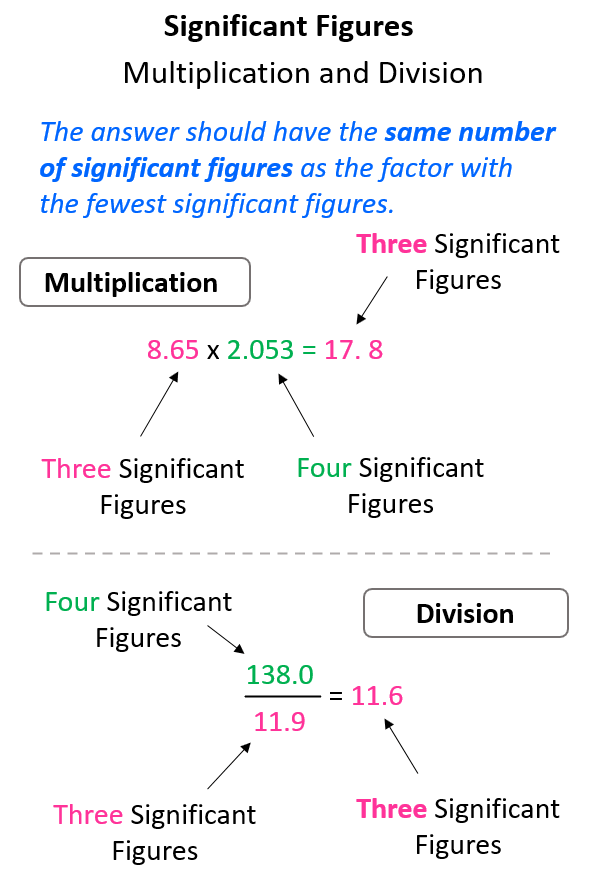
Sig Fig Rules Chart 30.00 has 4 significant figures (3, 0, 0 and 0) and 2 decimals. 0.0025 has 2 significant figures (2 and 5) and 4 decimals. sig fig calculator operators. you can use the following operators and functions with our calculator: addition ( ), subtraction ( ), division ( or ÷ ) and multiplication ( * or × ). plus exponent ( ^ ). Explicitly state the number of significant figures (the abbreviation s.f. is sometimes used): for example "20 000 to 2 s.f." or "20 000 (2 sf)". state the expected variability (precision) explicitly with a plus–minus sign, as in 20 000 ± 1%. this also allows specifying a range of precision in between powers of ten.

Comments are closed.