Solubility And Concentration Worksheet

Concentration And Solubility Worksheet Concentration: the abundance of a constituent divided by the total volume of a mixture. e.g the amount of salt in a water solution. solubility: a property referring to the ability for a given substance, the solute, to dissolve in a solvent, such as water. the solubility of a majority of solid substances increases as temperature increases. Rarely, however, do we want to use the acid or base in its concentrated form – we must dilute it to the exact concentration we want. use the table below to help you answer the following questions. as always, show your work! concentrated solution. molarity of concentrate (m) acetic acid, glacial (ch3cooh) 17.5.
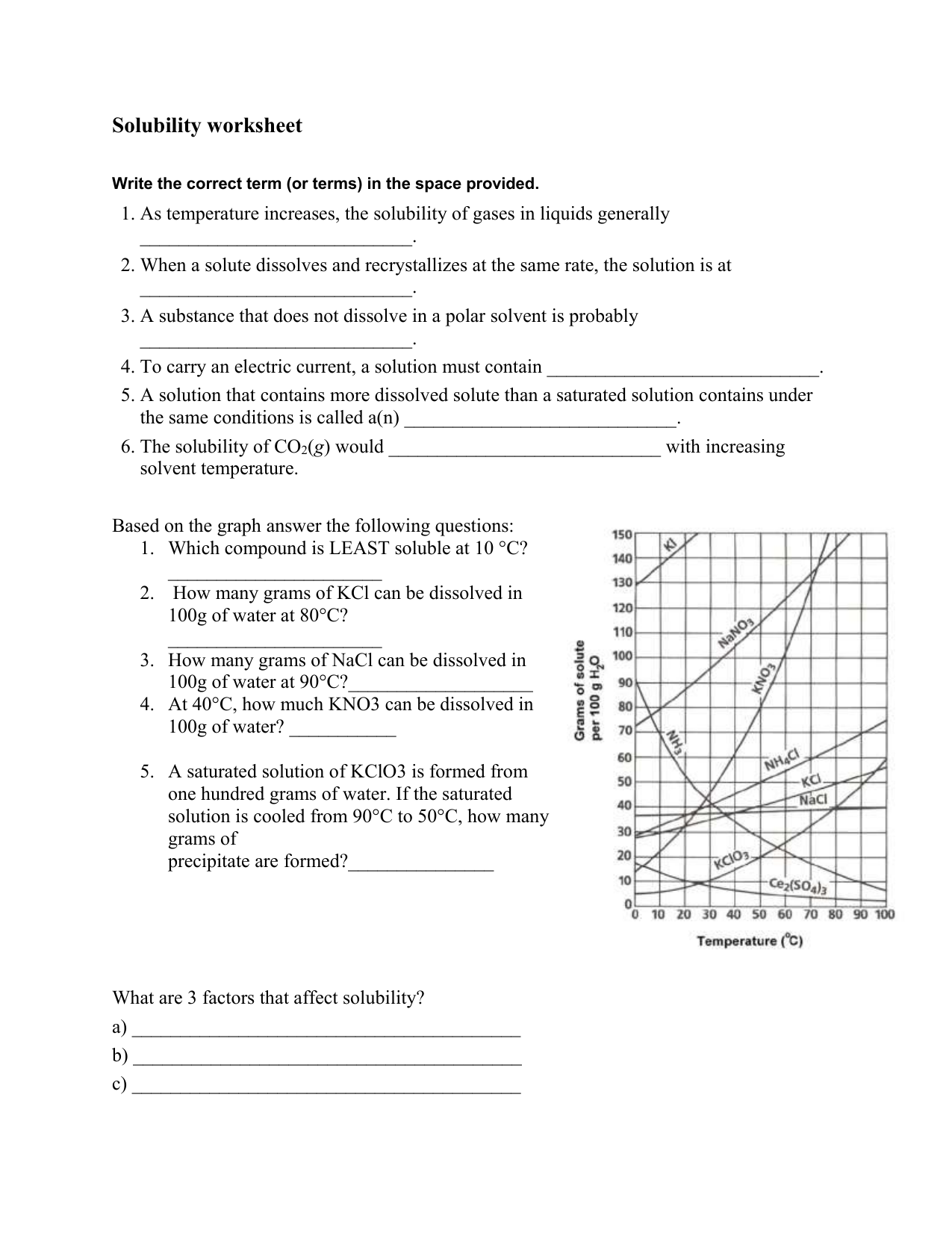
Solubility Worksheet Solubility and concentration. difficulty level: basic | created by: ck 12. last modified: aug 21, 2014. read. resources. details. determine if the following statements are true or false. read this passage from the text and answer the questions that follow. thoroughly answer the question below. Solutions are made up of solvents and solutes. the solvent makes up the largest amount of a solution. it is the substance into which another substance dissolves. the solute makes up a smaller amount of a solution. it is the substance that dissolves into another substance. the more solute there is in a solution, the higher the. Use the solubility cure below to answer the following questions: 34) which salt is least soluble at 20 °c? 35) how many grams of kbr can be dissolved in 100g of water at 60°c? 36) how many grams of nacl can be dissolved in 100g of water at 100°c? 37) at 40°c, 180g of naclo 3 is dissolved in 100g of water. is this solution. Calculate the solubility in moles l of each of three salts and the concentration of the cations in mg ml in each of the saturated solutions. agcn a g c n with ksp = 2.0 ×10‐12 k s p = 2.0 × 10 ‐ 12. baso4 b a s o 4 with ksp = 1.5 ×10‐9 k s p = 1.5 × 10 ‐ 9. fes f e s with ksp = 3.7 ×10‐19 k s p = 3.7 × 10 ‐ 19.
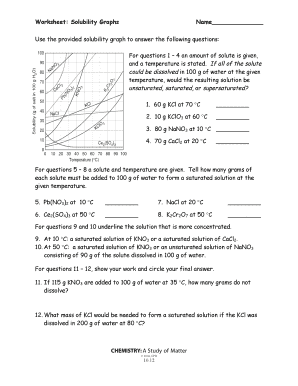
Solubility And Concentration Worksheet Use the solubility cure below to answer the following questions: 34) which salt is least soluble at 20 °c? 35) how many grams of kbr can be dissolved in 100g of water at 60°c? 36) how many grams of nacl can be dissolved in 100g of water at 100°c? 37) at 40°c, 180g of naclo 3 is dissolved in 100g of water. is this solution. Calculate the solubility in moles l of each of three salts and the concentration of the cations in mg ml in each of the saturated solutions. agcn a g c n with ksp = 2.0 ×10‐12 k s p = 2.0 × 10 ‐ 12. baso4 b a s o 4 with ksp = 1.5 ×10‐9 k s p = 1.5 × 10 ‐ 9. fes f e s with ksp = 3.7 ×10‐19 k s p = 3.7 × 10 ‐ 19. Nacl(aq) = na (aq) cl – (aq) agcl(s) = agcl(s) this page titled 5a: solubility and solution reactions (worksheet) is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by robert carter. so far, we have considered stoichiometric relationships on the basis of masses and moles. often it is more convenient to add. This worksheet provides a check for student understanding after a lesson about solutions, concentration, and solubility. use this as guided notes to follow along with a lesson or as a pre lab assignment before doing a lab about solubility and saturated solutions.

Solubility And Concentration Simulation Worksheet Print And Digital Nacl(aq) = na (aq) cl – (aq) agcl(s) = agcl(s) this page titled 5a: solubility and solution reactions (worksheet) is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by robert carter. so far, we have considered stoichiometric relationships on the basis of masses and moles. often it is more convenient to add. This worksheet provides a check for student understanding after a lesson about solutions, concentration, and solubility. use this as guided notes to follow along with a lesson or as a pre lab assignment before doing a lab about solubility and saturated solutions.

Comments are closed.