Solute Solvent And Solution What Is A Solution Science Video For Kids

Solute Solvent And Solution What Is A Solution Science Video For #scienceforkids #science #education #learningjunction #solution #chemistrya solution is a specific type of mixture where one substance is dissolved into anot. Learn the definition and difference of solution, solvent and solute with this chemistry science video for kids. visit the website for more resources.

Year 7 Science Lesson Solute Solvent Solution Edplace Youtube In this animated lecture, i will teach you about solution, solvent and solute in chemistry. also, you will learn that why water is called universal solvent. 📈 solubility measures how much solute can be dissolved in a liter of solvent, leading to a saturated solution when the limit is reached. 🌡️ concentration of a solution is determined by the proportion of solute to solvent; high solute makes it concentrated, low solute makes it dilute. Solute the solute is the substance that is being dissolved by another substance. in the example above, the salt is the solute. solvent the solvent is the substance that dissolves the other substance. in the example above, the water is the solvent. a solution is a type of homogeneous mixture. dissolving. a solution is made when one substance. Making solutions. a simple solution is basically two substances that are evenly mixed together. one of them is called the solute and the other is the solvent. a solute is the substance to be dissolved (sugar). the solvent is the one doing the dissolving (water). as a rule of thumb, there is usually more solvent than solute.
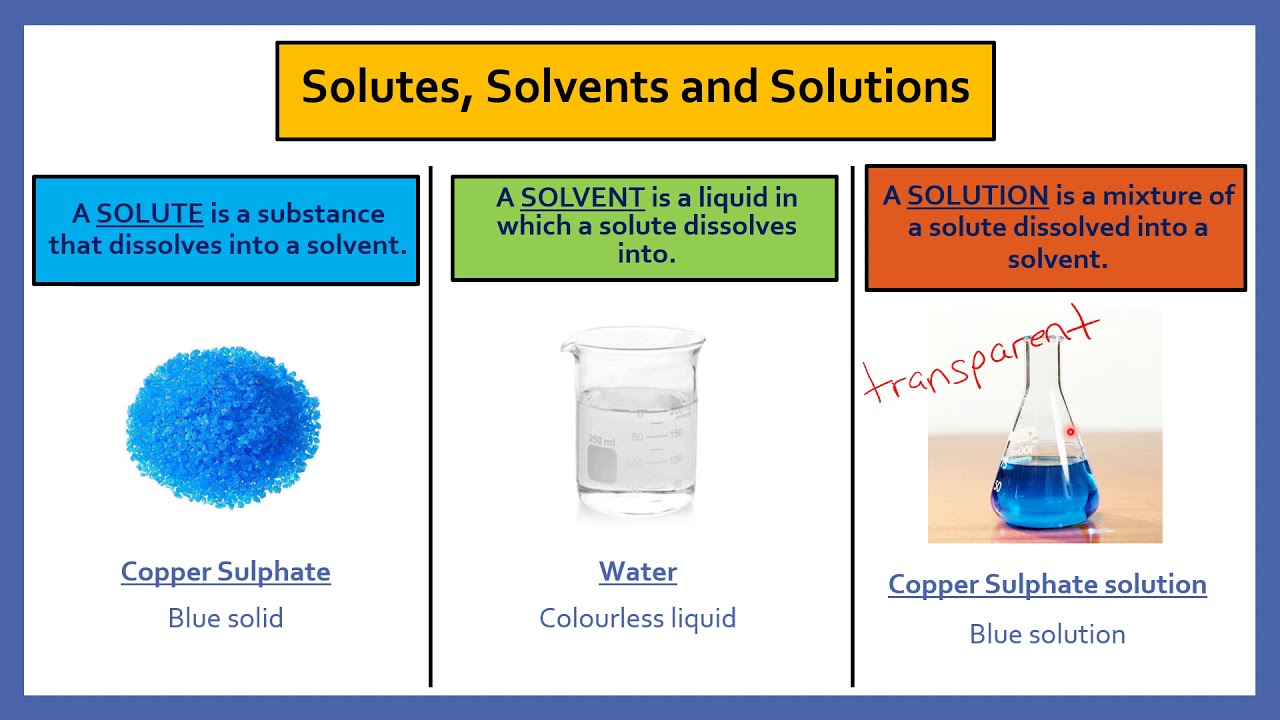
Solutes Solvents And Solutions Remote 1 Youtube Solute the solute is the substance that is being dissolved by another substance. in the example above, the salt is the solute. solvent the solvent is the substance that dissolves the other substance. in the example above, the water is the solvent. a solution is a type of homogeneous mixture. dissolving. a solution is made when one substance. Making solutions. a simple solution is basically two substances that are evenly mixed together. one of them is called the solute and the other is the solvent. a solute is the substance to be dissolved (sugar). the solvent is the one doing the dissolving (water). as a rule of thumb, there is usually more solvent than solute. Solvent the liquid in a solution which dissolves the solute. for example, the solvent in sea water is water. dissolve the process when a solute is mixed with a solvent and the solute breaks into. Solutes and solvents. a solution forms when a substance dissolves, or breaks apart, into another substance. the substance that dissolves to form a solution is called a solute. the substance in which a solute will dissolve is called a solvent. in a sugar water solution, sugar is the solute and water is the solvent. the water dissolves the sugar.

Solvent Solute And Solution Science For Grade 4 Science Solvent the liquid in a solution which dissolves the solute. for example, the solvent in sea water is water. dissolve the process when a solute is mixed with a solvent and the solute breaks into. Solutes and solvents. a solution forms when a substance dissolves, or breaks apart, into another substance. the substance that dissolves to form a solution is called a solute. the substance in which a solute will dissolve is called a solvent. in a sugar water solution, sugar is the solute and water is the solvent. the water dissolves the sugar.

Solute Solvent And Solutions Class 4 Youtube

Comments are closed.