Solved 1 A Provider Orders Azacitidine 75mg M2 Iv For 7 Chegg

Solved 1 A Provider Orders Azacitidine 75mg M2 Iv For 7 Chegg To calculate the dose of azacitidine for the patient, we need to determine the body sur 1. a provider orders azacitidine 75mg m2. iv, for 7 days. the patient weighs 80 kg and is 176 cm in height. mg 148mg 149mg question 30 1 pts 32. a provider orders mitoxantrone 12mg m2,iv, one dose only today. the patient weighs 52 kg and is 147 cm in height. 2.5 mg kg iv or subcutaneously daily for 7 days in a 28 day cycle. 1 year and older and weighing 10 kg or greater: 75 mg m2 iv or subcutaneously daily for 7 days in a 28 day cycle. duration of therapy: 3 to 6 cycles. comments: premedicate patients for nausea and vomiting prior to each dose for the first two cycles.

Solved The Healthcare Provider Orders 75mg п їof A Drug Npb Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: a provider orders amikacin 5 mg kg d, iv, every 12 hours. the patient weighs 87 kg. the pharmacy provides amikacin in a vial that contains 50 mg ml. round to the nearest tenth ml. The resulting solution will contain azacitidine 10 mg ml. the solution should be clear. withdraw the required amount of azacitidine for injection solution to deliver the desired dose and inject into a 50 to 100 ml infusion bag of either 0.9% sodium chloride injection, usp or lactated ringer’s injection, usp. 100mg single dose vial (vidaza) juvenile myelomonocytic leukemia. indicated for juvenile myelomonocytic leukemia (jmml) in children aged ≥1 month. ≥1 month to <1 year or weighs <10 kg: 2.5 mg kg iv qday for 7 days in a 28 day cycle. ≥1 year and weighs ≥10 kg: 75 mg m iv qday for 7 days in a 28 day cycle. No dose modification with first cycle. commence azacitidine at 100% dose in the first cycle regardless of baseline haematology counts. platelet transfusions may be needed. haematological toxicity is defined as the lowest count reached in a given cycle (nadir) if platelets ≤ 50 x 109 l and or absolute neutrophil count (anc) ≤ 1 x 109 l.

Solved 4 A Provider Orders Bivalirudin 0 75mg Kg Iv One Chegg 100mg single dose vial (vidaza) juvenile myelomonocytic leukemia. indicated for juvenile myelomonocytic leukemia (jmml) in children aged ≥1 month. ≥1 month to <1 year or weighs <10 kg: 2.5 mg kg iv qday for 7 days in a 28 day cycle. ≥1 year and weighs ≥10 kg: 75 mg m iv qday for 7 days in a 28 day cycle. No dose modification with first cycle. commence azacitidine at 100% dose in the first cycle regardless of baseline haematology counts. platelet transfusions may be needed. haematological toxicity is defined as the lowest count reached in a given cycle (nadir) if platelets ≤ 50 x 109 l and or absolute neutrophil count (anc) ≤ 1 x 109 l. Iv: reconstitute vial with 10 ml swfi to form a 10 mg ml solution; vigorously shake or roll vial until solution is dissolved and clear. mix in 50 to 100 ml of ns or lactated ringer's injection for infusion. subq: slowly add 4 ml swfi to each vial, resulting in a concentration of 25 mg ml. Background: in the aza 001 trial, azacitidine (aza; 75 mg m 2 per day for 7 consecutive days of every 28 day cycle) demonstrated improved survival compared with conventional care regimens in patients with high risk myelodysplastic syndromes (mds). although a 7 day regimen of aza is the standard treatment of high risk mds, it is difficult to.
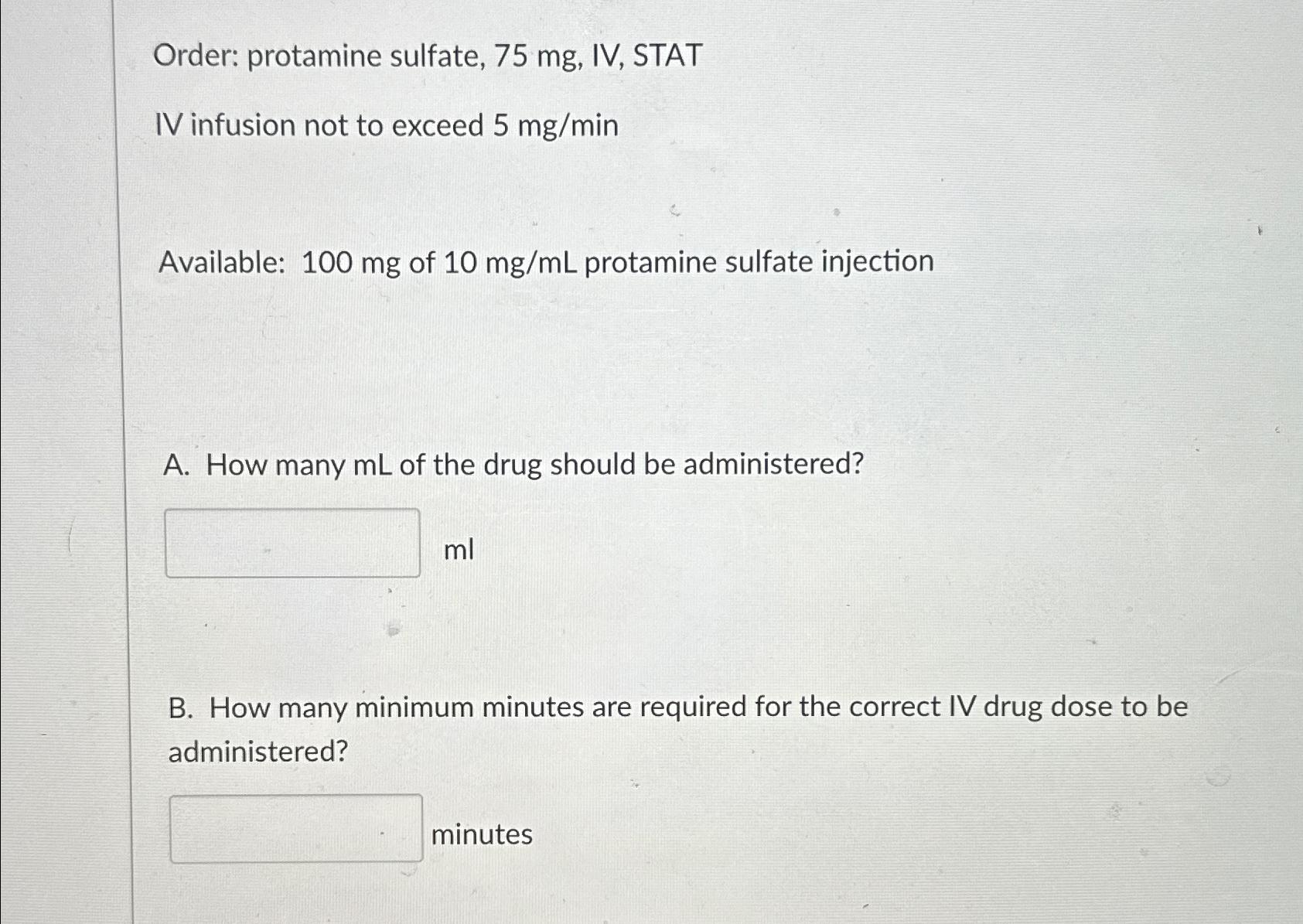
Solved Order Protamine Sulfate 75mg п їiv п їstativ Infusion Chegg Iv: reconstitute vial with 10 ml swfi to form a 10 mg ml solution; vigorously shake or roll vial until solution is dissolved and clear. mix in 50 to 100 ml of ns or lactated ringer's injection for infusion. subq: slowly add 4 ml swfi to each vial, resulting in a concentration of 25 mg ml. Background: in the aza 001 trial, azacitidine (aza; 75 mg m 2 per day for 7 consecutive days of every 28 day cycle) demonstrated improved survival compared with conventional care regimens in patients with high risk myelodysplastic syndromes (mds). although a 7 day regimen of aza is the standard treatment of high risk mds, it is difficult to.

Comments are closed.