Solved Calculate The Total Energy Required In Joules To Chegg
Solved Calculate The Total Energy Required In Joules To Chegg Calculate the total energy required (in joules) to bring 100.00 g of compound “x” (mw =100.00 g mol) from 85.00 degrees c to 220.00 degrees c. correct answer is 120,484 j. need help understanding how can i get this answer. Calculate the total energy required (in joules) to bring 360.00 g of compound "x" (mw=140.0 g mol) from 60.00 degrees c to 245.00 degrees c data is given in picture your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on.

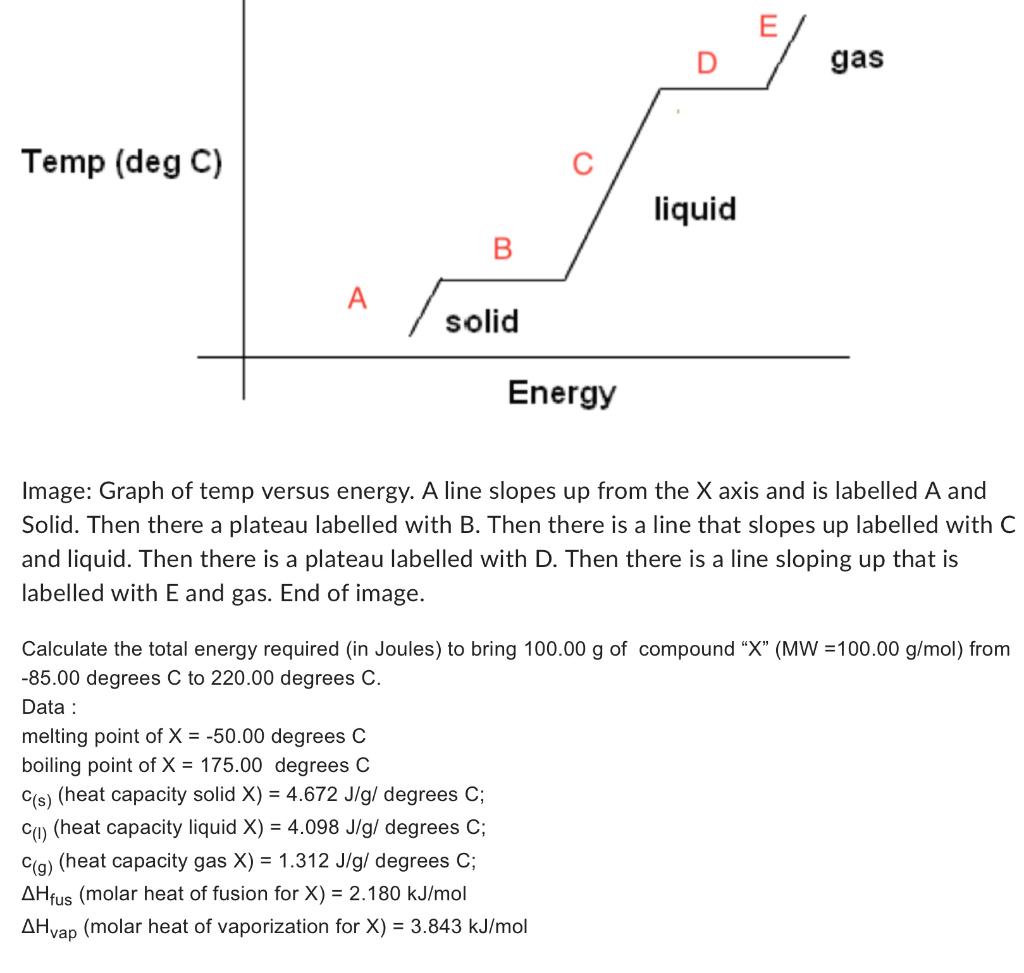
Solved Calculate The Total Energy Required In Joules To Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. question: calculate the total energy required (in joules) to bring 380.00 g of compound “x” (mw =110.00 g mol) from 90.00 degrees c to 215.00 degrees c. the correct answer is 459,114 j. The equation that relates heat (q) (q) to specific heat (cp) (c p), mass (m) (m), and temperature change (Δt) (Δ t) is shown below. q = cp × m × Δt q = c p × m × Δ t. the heat that is either absorbed or released is measured in joules. the mass is measured in grams. the change in temperature is given by Δt = tf −ti Δ t = t f − t i. The total energy required is the sum of the energy to heat the 10 °c ice to 0 °c ice, melting the 0 °c ice into 0 °c water, heating the water to 100 °c, converting 100 °c water to 100 °c steam and heating the steam to 150 °c. to get the final value, first calculate the individual energy values and then add them up. step 1:. Question: calculate the total energy (in joules) needed to convert 15.0 grams of liquid ethanol at 25 0c to gas at 78 0c (boiling point). the specific heat of ethanol is 2.46 j g 0c and the heat of vaporization is 841 j g.

Solved Calculate The Quantity Of Energy In Joules Required Chegg The total energy required is the sum of the energy to heat the 10 °c ice to 0 °c ice, melting the 0 °c ice into 0 °c water, heating the water to 100 °c, converting 100 °c water to 100 °c steam and heating the steam to 150 °c. to get the final value, first calculate the individual energy values and then add them up. step 1:. Question: calculate the total energy (in joules) needed to convert 15.0 grams of liquid ethanol at 25 0c to gas at 78 0c (boiling point). the specific heat of ethanol is 2.46 j g 0c and the heat of vaporization is 841 j g. δq = mcδt δ q = m c δ t. now all you have to do is to use this formula to convert 8.49g of water at 24.5°c to water at 0°c. but after this you need to use the concept of latent heat i.e. heat you need to remove to convert water at 0°c to ice at 0°c.as this value is given as 6.01 kj mol you will have to remove that much amount of heat. F =c λ. where c is the speed of light, f the frequency and λ the wavelength. if you know the frequency, or if you just calculated it, you can find the energy of the photon with planck's formula: e = h × f. where h is the planck's constant: h = 6.62607015e 34 m² · kg s. 3. remember to be consistent with the units!.

Comments are closed.