Solved Learning Goal To Calculate The Total Energy Required Chegg

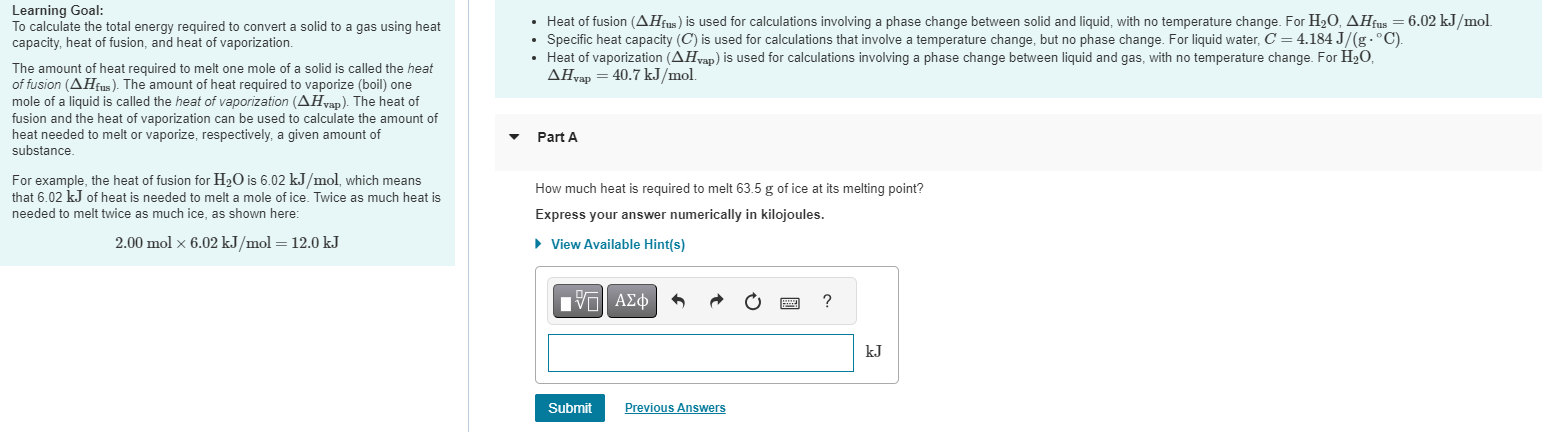
Solved Learning Goal To Calculate The Total Energy Required Chegg The heat. to calculate the total energy required to convert a solid to a gas using heat capacity, heat of fusion, and heat of vaporization. the amount of heat required to melt one mole of a solid is called the heat of fusion (? h fus). the amount of heat required to vaporize (boil) one mole of a liquid is called the heat of vaporization (? h vap). The equation that relates heat (q) (q) to specific heat (cp) (c p), mass (m) (m), and temperature change (Δt) (Δ t) is shown below. q = cp × m × Δt q = c p × m × Δ t. the heat that is either absorbed or released is measured in joules. the mass is measured in grams. the change in temperature is given by Δt = tf −ti Δ t = t f − t i.
Solved Learning Goal To Calculate The Total Energy Required Chegg Alternatively, we can use equation 13.7 to find v orbit v orbit and calculate the kinetic energy directly from that. the total energy required is then the kinetic energy plus the change in potential energy found in example 13.8. solution from equation 13.9, the total energy of the soyuz in the same orbit as the iss is. Set this total work equal to the change in kinetic energy and solve for any unknown parameter. check your answers. if the object is traveling at a constant speed or zero acceleration, the total work done should be zero and match the change in kinetic energy. if the total work is positive, the object must have sped up or increased kinetic energy. This is a result of the law of conservation of energy, which says that, in a closed system, total energy is conserved—that is, it is constant. using subscripts 1 and 2 to represent initial and final energy, this law is expressed as. k e 1 p e 1 = k e 2 p e 2 . either side equals the total mechanical energy. The total energy required is the sum of the energy to heat the 10 °c ice to 0 °c ice, melting the 0 °c ice into 0 °c water, heating the water to 100 °c, converting 100 °c water to 100 °c steam and heating the steam to 150 °c. to get the final value, first calculate the individual energy values and then add them up. step 1:.

Solved Learning Goal To Calculate The Total Energy Required Chegg This is a result of the law of conservation of energy, which says that, in a closed system, total energy is conserved—that is, it is constant. using subscripts 1 and 2 to represent initial and final energy, this law is expressed as. k e 1 p e 1 = k e 2 p e 2 . either side equals the total mechanical energy. The total energy required is the sum of the energy to heat the 10 °c ice to 0 °c ice, melting the 0 °c ice into 0 °c water, heating the water to 100 °c, converting 100 °c water to 100 °c steam and heating the steam to 150 °c. to get the final value, first calculate the individual energy values and then add them up. step 1:. How is the energy required to place a satellite in orbit calculated? the energy required to place a satellite in orbit is calculated using the formula e = g x m x m r, where e is the energy, g is the gravitational constant, m is the mass of the planet, m is the mass of the satellite, and r is the distance between the satellite and the center. Learning goal: to calculate the total energy required to convert a solid to a gas using heat capacity, heat of fusion, and heat of vaporization. the amount of heat required to melt one mole of a solid is called the heat of fusion (Δhfus ). the amount of heat required to vaporize (boil) one mole of a liquid is called the heat of vaporization.

Solved Learning Goal To Calculate The Total Energy Required Chegg How is the energy required to place a satellite in orbit calculated? the energy required to place a satellite in orbit is calculated using the formula e = g x m x m r, where e is the energy, g is the gravitational constant, m is the mass of the planet, m is the mass of the satellite, and r is the distance between the satellite and the center. Learning goal: to calculate the total energy required to convert a solid to a gas using heat capacity, heat of fusion, and heat of vaporization. the amount of heat required to melt one mole of a solid is called the heat of fusion (Δhfus ). the amount of heat required to vaporize (boil) one mole of a liquid is called the heat of vaporization.

Comments are closed.