Solved Qualitative Analysis Of Group Iii Cations Qualitative ођ
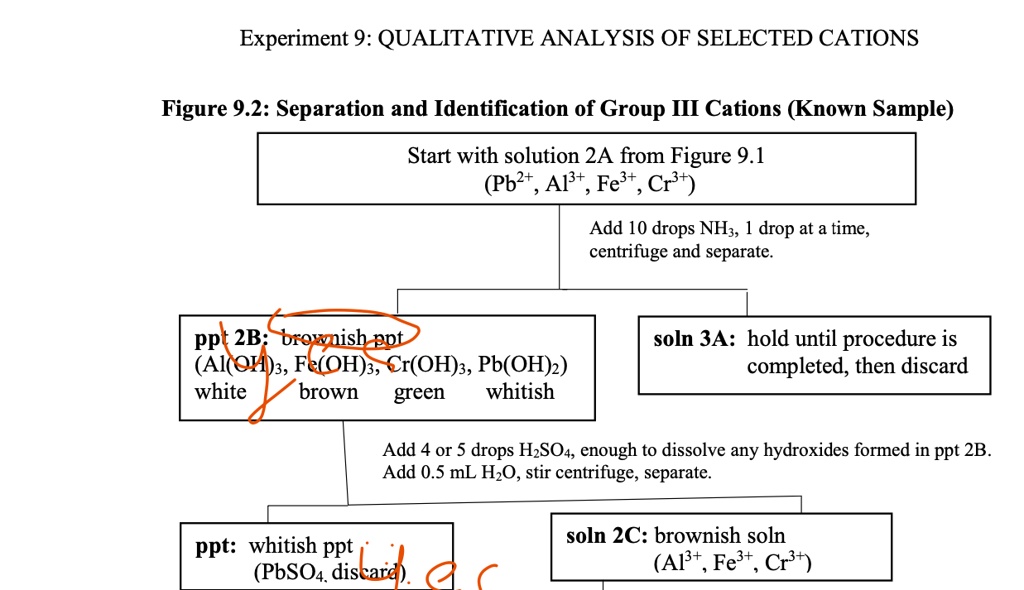
Solved Experiment 9 Qualitative Analysis Of Selected Cations Figure 9 Part b: analysis and identification of group iii cations in an unknown sample. obtain a test tube which contains a mixture of group iii cations. record the id code of the sample on your report form. transfer 2.0 ml of the above mixture into a 30 ml beaker and repeat the procedure used in part a. Qualitative analysis of group iii cations the four group iii cations in this lab are cr 3, al 3, fe 3, and ni 2. the first step in analysis involves separating the ions into two subgroups by treating the solution with naoh and naocl. the hypochlorite ion oxidizes cr(iii) to a higher, more stable oxidation state (namely cr(vi)) which is soluble:.
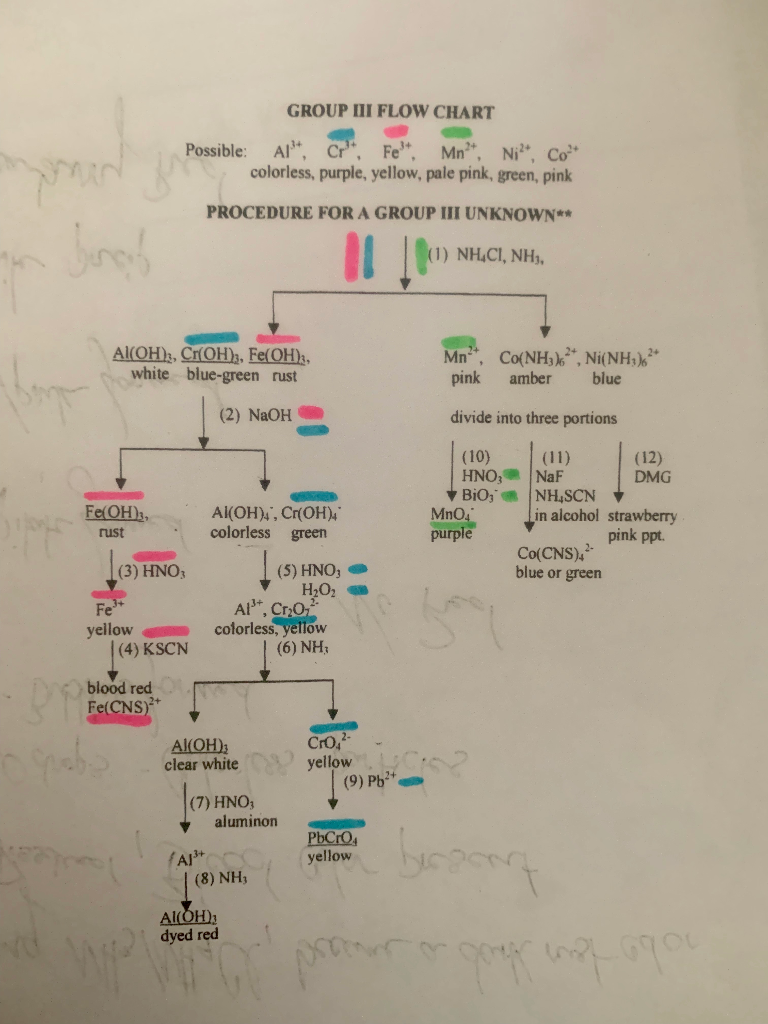
Solved Qualitative Analysis Group Iii The Cations In My Chegg Chemistry. chemistry questions and answers. experiment 38 advance study assignment: qualitative analysis of group iii cations 1. prepare a flow diagram for the separation and identification of the ions in group iii and put it on the data page for this experiment. 2. write balanced net ionic equations for the following reactions: a. Chemistry. chemistry questions and answers. prelab assignment: qualitative analysis of group iii cations given the very low value of ks for cr (oh)3, a precipitate of cr (oh)s would be expected if only 6 m naoh were added to the mixture of group l cations in the first step of the 1. 2. a solution may contain one or more of the group ill cations. Qualitative analysis of group iii cations qualitative analysis is a method for identifying the elements present in a unknown mixture. for this purpose, metal cations are broadly classified into three groups based upon their solubilities. the group i cations, pb2 , ag , and hg22 , are those which form insoluble chlorides. Step 1: precipitation of iii (a) cations as hydroxide to the filtrate, add few drops of nitric acid hno3 to convert ferrous fe2 into ferric fe3 . then add solid ammonium chloride nh4cl3 and ammonia nh3 solution and boil. on boiling you will get precipitate of iii (a) cations which may contains fe (oh)3, cr (oh)3, al (oh)3 and a little mno (oh)2.

Solved Qualitative Analysis Of Group Iii Cations Qualitativeо Qualitative analysis of group iii cations qualitative analysis is a method for identifying the elements present in a unknown mixture. for this purpose, metal cations are broadly classified into three groups based upon their solubilities. the group i cations, pb2 , ag , and hg22 , are those which form insoluble chlorides. Step 1: precipitation of iii (a) cations as hydroxide to the filtrate, add few drops of nitric acid hno3 to convert ferrous fe2 into ferric fe3 . then add solid ammonium chloride nh4cl3 and ammonia nh3 solution and boil. on boiling you will get precipitate of iii (a) cations which may contains fe (oh)3, cr (oh)3, al (oh)3 and a little mno (oh)2. A red. color of [fe(scn)]2 ion confirms the presence of fe3 in test solution. 9. test for al3 to the al(oh)4 solution from step 7, add 6 m hcl until acidic to litmus (blue → red) then add ~0.5 ml excess. add 1 3 drops of aluminon reagent and ~1 2 1 ml of h2o and mix. add 6 m nh3 until just basic to litmus. Other additional steps are needed to separate metal ions that precipitate together. 18.9: qualitative cation analysis is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. in qualitative analysis, the identity, not the amount, of metal ions present in a mixture is determined.

Solved Qualitative Analysis Of Group Iii Cations You Will Chegg A red. color of [fe(scn)]2 ion confirms the presence of fe3 in test solution. 9. test for al3 to the al(oh)4 solution from step 7, add 6 m hcl until acidic to litmus (blue → red) then add ~0.5 ml excess. add 1 3 drops of aluminon reagent and ~1 2 1 ml of h2o and mix. add 6 m nh3 until just basic to litmus. Other additional steps are needed to separate metal ions that precipitate together. 18.9: qualitative cation analysis is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. in qualitative analysis, the identity, not the amount, of metal ions present in a mixture is determined.
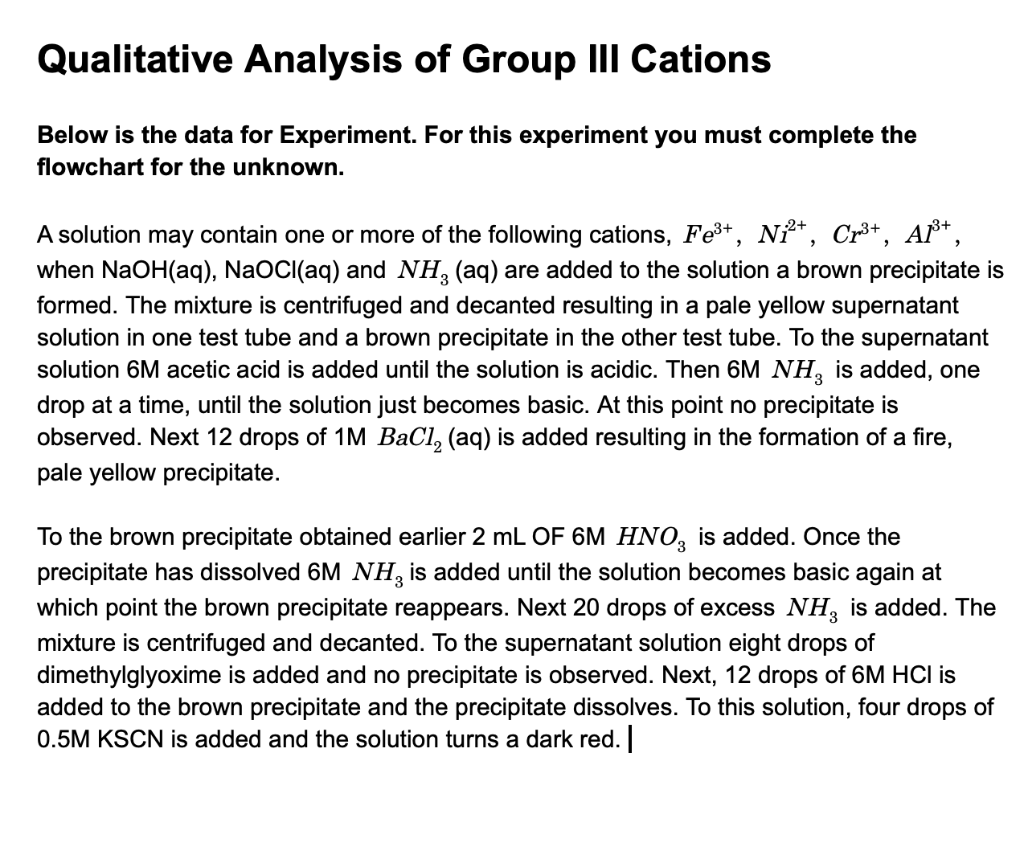
Solved Qualitative Analysis Of Group Iii Cations Below Is Chegg

Comments are closed.