Solved Question 3 Determine The Total Energy Required To Chegg

Solved Question 3 Determine The Total Energy Required To Chegg Physics questions and answers. 13) a physics class of 27 students taking an exam has a power output per student of about 225 w. (a) (3 points): calculate the total energy ouput of the all the students. (b) (5 points) assume the initial temperature of the room is 22 \deg c and that its dimensions are 6.8 m by 15.2 m by 3.0m. Step 1. to calculate the total energy needed to melt the ice, heat it to boiling, boil it, and heat the stea q.7. calculate the total energy needed to melt 3.0 g of ice at 0.0∘c, heat it to boiling, boil it, and heat the steam to 125∘c ? (note: the specific heat of steam is 2.01 j g∘c ).

Solved Question 3 Determine The Total Energy Required To Chegg Question: a cylindrical vessel of 5 cm radius contains 300 ml oil and 600 ml water separated in two phases. the system is stirred until the oil is dispersed in the water in the form of drops of 1 \mu m radius (assume that all the drops have the same size). a) considering that the surface energy of the interface oil water is 35 mj. Total amount of heat energy required = ? heat energy taken by ice at 0° c to convert into water at 0° c. = m x l. = 200 × 336. = 67200 j. heat energy taken by water to raise the temperature from 0° c to 100° c. = m x c x change in temperature. = 200 × 4.2 × (100 0) = 200 × 4.2 × 100. The variational method: solved problems. the variational method: solved problems. 1. compute the variational upper bounds for the ground state energy of a particle in the linear potential v(x) = fi j x j and in the quartic potential v(x) = fi x4. using the gaussian trial wavefunction ˆ(x) = a e¡bx2: (a) calculate the normalization constant a. A) sign convention is not important for internal resultants since they are squared in the energy 6. internal resultants →strain energy in each segment →total strain energy (u = u 1 u 2 u 3 …) a) sanity warning: do not expand the squared terms in the integrals!!! 7. for indeterminate problems: find all the reactions a) take 0 = ∂u.
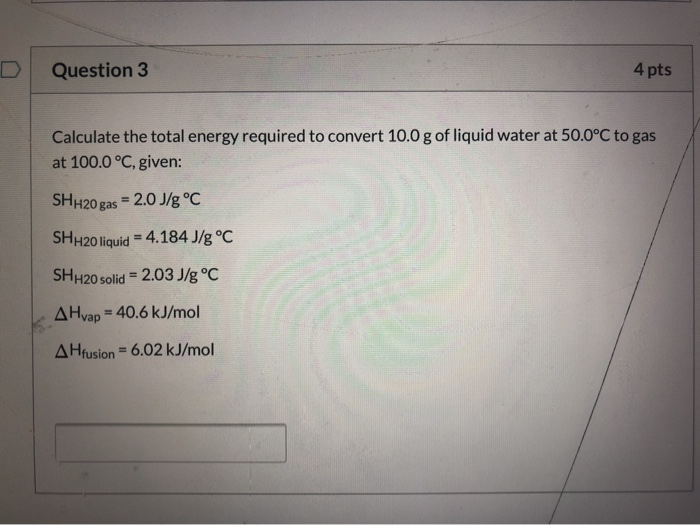
Solved Question 3 4 Pts Calculate The Total Energy Required C The variational method: solved problems. the variational method: solved problems. 1. compute the variational upper bounds for the ground state energy of a particle in the linear potential v(x) = fi j x j and in the quartic potential v(x) = fi x4. using the gaussian trial wavefunction ˆ(x) = a e¡bx2: (a) calculate the normalization constant a. A) sign convention is not important for internal resultants since they are squared in the energy 6. internal resultants →strain energy in each segment →total strain energy (u = u 1 u 2 u 3 …) a) sanity warning: do not expand the squared terms in the integrals!!! 7. for indeterminate problems: find all the reactions a) take 0 = ∂u. Use rydberg's equation to solve for the wavelength required for the electronic transition from n = 3 to n = 5 in the hydrogen atom. from the wavelength you can then find the energy of transition. step i: find wavelength as: mathematically, rydberg's equation is: 1 λ = r (1 n initial 2 − 1 n final 2), where. λ = the wavelength of photon. Electrical engineering questions and answers; suppose x(t)=sinc(t)2. [20 points](a) use parseval's relationship to determine the energy ex.(b) determine the energy in the frequency band 0,1. what percentage isthis of the total energy?(c) determine the absolute bandwidth.(d) determine the 3 db bandwidth.

Solved Determine The Total Energy Required To Assemble The Chegg Use rydberg's equation to solve for the wavelength required for the electronic transition from n = 3 to n = 5 in the hydrogen atom. from the wavelength you can then find the energy of transition. step i: find wavelength as: mathematically, rydberg's equation is: 1 λ = r (1 n initial 2 − 1 n final 2), where. λ = the wavelength of photon. Electrical engineering questions and answers; suppose x(t)=sinc(t)2. [20 points](a) use parseval's relationship to determine the energy ex.(b) determine the energy in the frequency band 0,1. what percentage isthis of the total energy?(c) determine the absolute bandwidth.(d) determine the 3 db bandwidth.

Comments are closed.