Solved The Plateau Portion Of The Cooling Curve Is Characteristic For
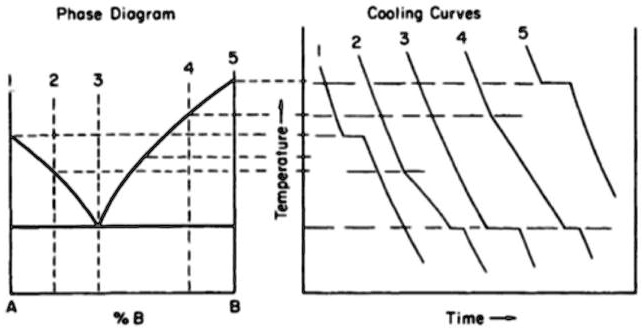
Solved The Plateau Portion Of The Cooling Curve Is Characteristic For The plateau portion of cooling curve 3 is characteristic for the liquid to crystaltransformations:a. at the freezing point of component a. b. at the freezing point of component b. c. at the freezing point of melt. d. at the eutectic temperature of melt. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa.

15 The Plateau Portion Of Cooling Curve 3 Is Chegg The plateau portion of the cooling curve is characteristic for the liquid to crystal transformations: a. at the freezing point of component a, b. at the freezing point of component b, c. at the freezing point of the melt, d. at the eutectic temperature of the melt. phase diagram cooling curves temperature a %b time. The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The resulting cooling curve shows the two stages of solidification with a section of reduced gradient where a single phase is solidifying and a plateau where eutectic is solidifying. by taking a series of cooling curves for the same system over a range of compositions the liquidus and solidus temperatures for each composition can be determined. State changes are visible as plateaus on cooling curves. as it cools, a substance transfers energy into the surroundings by heating and it may condense or freeze. energy is released to the surroundings as a substance cools and this is observed as a decrease in substance temperature. graphs provide a visual representation of data for easier.
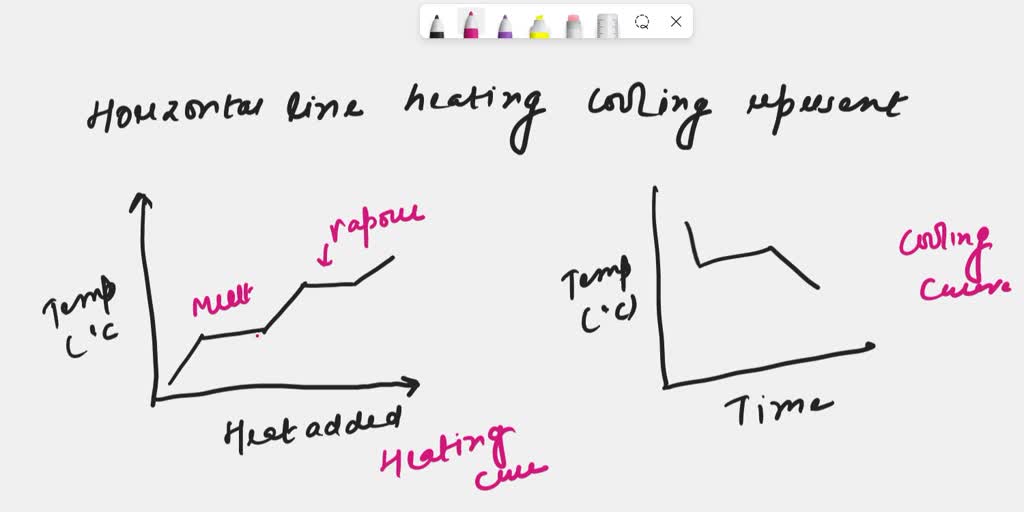
Solved A Plateau Horizontal Line On A Heating Or Cooling Curve The resulting cooling curve shows the two stages of solidification with a section of reduced gradient where a single phase is solidifying and a plateau where eutectic is solidifying. by taking a series of cooling curves for the same system over a range of compositions the liquidus and solidus temperatures for each composition can be determined. State changes are visible as plateaus on cooling curves. as it cools, a substance transfers energy into the surroundings by heating and it may condense or freeze. energy is released to the surroundings as a substance cools and this is observed as a decrease in substance temperature. graphs provide a visual representation of data for easier. A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate. A cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. the gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. the more heat is required to change the temperature of the substance, the slower it cools.

Solved A Plateau Horizontal Line On A Heating Or Cooling Curve A cooling curve for a sample that begins at the temperature and composition given by point a is shown in figure 8.10.1b 8.10. 1 b. figure 8.10.1 8.10. 1: (a) cooling of a two component system from liquid to solid. (b) cooresponding cooling curve for this process. as the sample cools from point a, the temperature will decrease at a rate. A cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. the gradient of the cooling curve is related to the heat capacity, the thermal conductivity of the substance, and the external temperature. the more heat is required to change the temperature of the substance, the slower it cools.

Solved On A Heating Curve A Plateau The Flat Portion Chegg

Comments are closed.