Strength Of Acids And Bases How To Find It Chemistry Teachoo

Strength Of Acids And Bases How To Find It Chemistry Teachoo The strength of an acid or base is measured on a scale of numbers from 0 14 called the ph scale. due to water mixed with the acid or base , solutions of both acid as well as base will certainly contain hydrogen molecules. except acids will have more hydrogen ions as compared to bases. the strength of an acid or base is nothing but the measure. You will also learn about the reactions of acids and bases with metals. lastly, you will look at the category of salts and their chemical properties as well as their uses in our day to day lives. chapter 2 of class 10, acids, bases and salts,is going to teach you about thenatureof solutions and various products that we use on a daily basis.
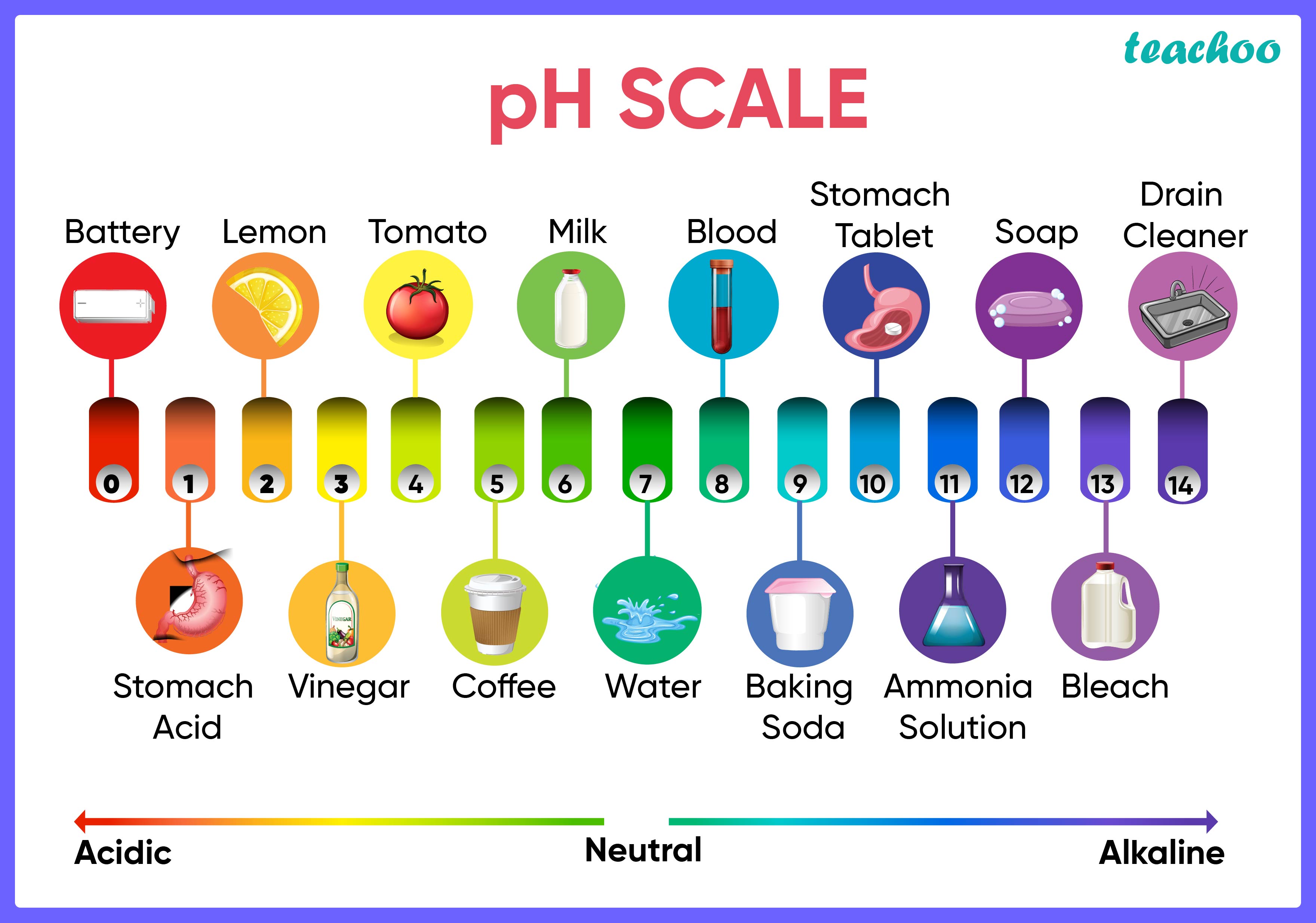
Strength Of Acids And Bases How To Find It Chemistry Teachoo Chapter 2 class 10 acids, bases and salts. indicators are substances which when added to an acid or base change colour and help decipher the nature of the substance being tested.there are three types of indicators:natural indicators:indicators organically found in nature. some common examples are:a.)litmus there are two types of litmus pape. This page titled 6.3: strength of acids and bases is shared under a public domain license and was authored, remixed, and or curated by muhammad arif malik. the strength of acids and bases, i.e., the extent of dissociation of the dissolved acid or base into ions in water is described. the relative strength of the acid conjugate base pair is also. The first six acids in figure 14.3.3 are the most common strong acids. these acids are completely dissociated in aqueous solution. the conjugate bases of these acids are weaker bases than water. when one of these acids dissolves in water, their protons are completely transferred to water, the stronger base. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in aqueous solution. ph = − log[h ] or. ph = − log[h3o ] figure 10.5.3 illus trates this relationship, along with some examples of various solutions.
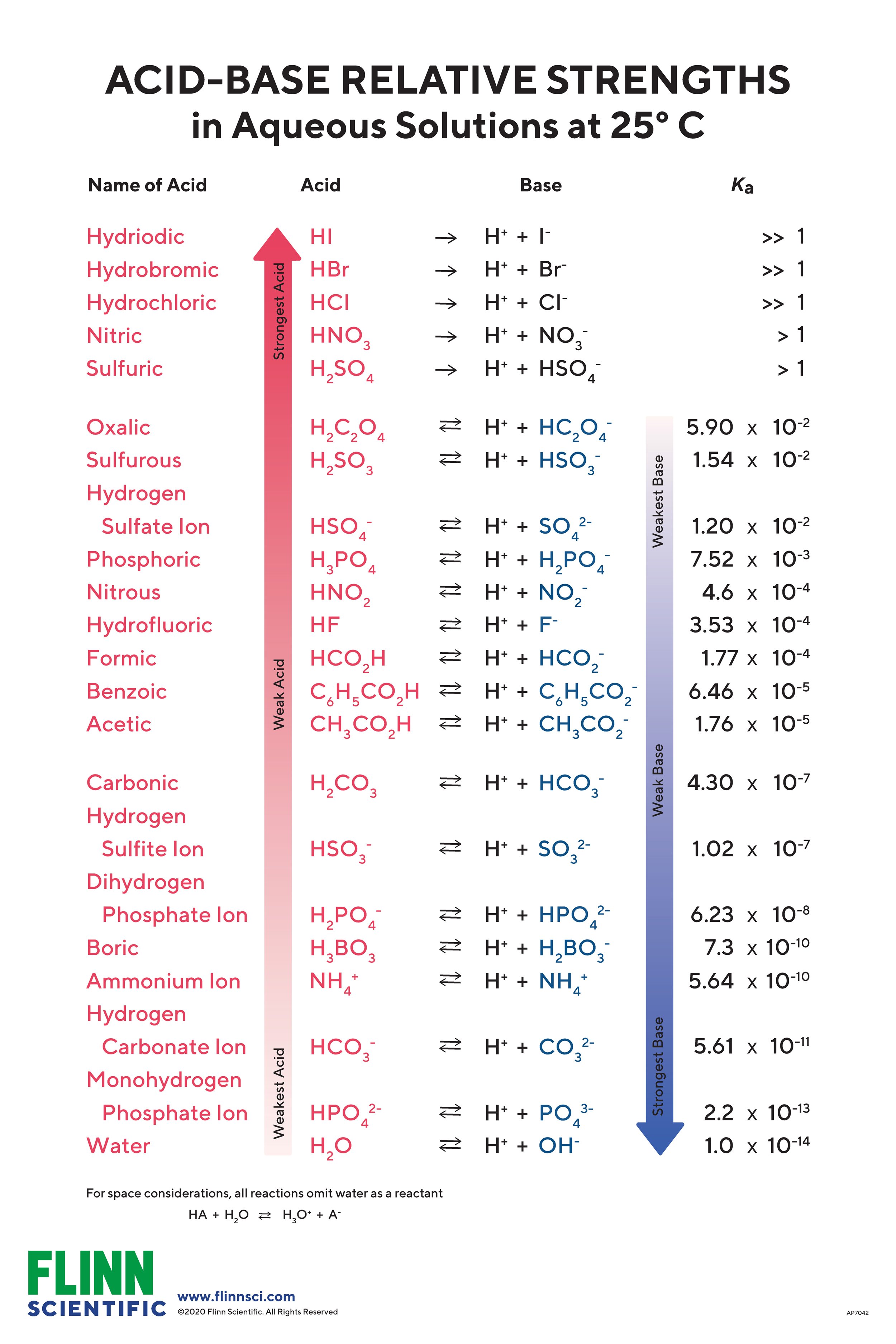
Acid Base Strength Charts For Chemistry The first six acids in figure 14.3.3 are the most common strong acids. these acids are completely dissociated in aqueous solution. the conjugate bases of these acids are weaker bases than water. when one of these acids dissolves in water, their protons are completely transferred to water, the stronger base. One qualitative measure of the strength of an acid or a base solution is the ph scale, which is based on the concentration of the hydronium (or hydrogen) ion in aqueous solution. ph = − log[h ] or. ph = − log[h3o ] figure 10.5.3 illus trates this relationship, along with some examples of various solutions. K a = k w k b or k b = k w k a. the inverse proportional relation between ka and kb means the stronger the acid or base, the weaker its conjugate partner. figure 14.7 illustrates this relation for several conjugate acid base pairs. figure 14.7 relative strengths of several conjugate acid base pairs are shown. Factors affecting acid strength. it depends on the strength of the h a bond. the weaker the bond, the lesser the energy required to break it. hence, the acid is strong. the polarity of the h a bond affects its acid strength. if the bond is highly polar, the proton tends to leave the molecule more easily, making it a strong acid.

How To Determine Acid And Base Strength Ft Professor Dave Youtube K a = k w k b or k b = k w k a. the inverse proportional relation between ka and kb means the stronger the acid or base, the weaker its conjugate partner. figure 14.7 illustrates this relation for several conjugate acid base pairs. figure 14.7 relative strengths of several conjugate acid base pairs are shown. Factors affecting acid strength. it depends on the strength of the h a bond. the weaker the bond, the lesser the energy required to break it. hence, the acid is strong. the polarity of the h a bond affects its acid strength. if the bond is highly polar, the proton tends to leave the molecule more easily, making it a strong acid.

Comments are closed.