Tendency To Decompose
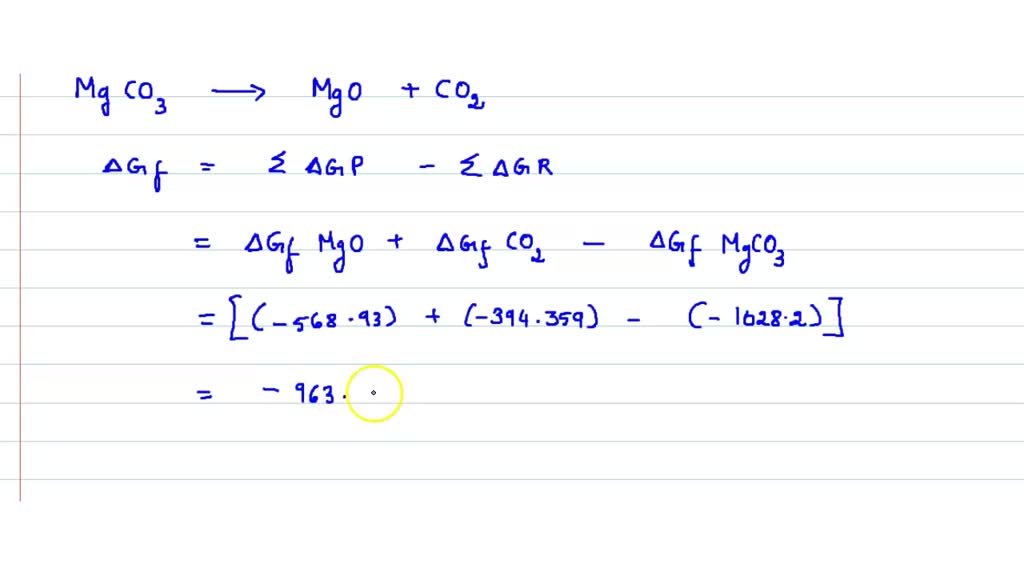
Using Data In Appendix L Calculate δr G Values For The Decomposition A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. the general form of a decomposition reaction is: ab → a b ab → a b. most decomposition reactions require an input of energy in the form of heat, light, or electricity. binary compounds are compounds composed of just two elements. (b) based on the information given, does nitrous oxide have a tendency to decompose into nitrogen and oxygen? (c) what is the value of kp for the reaction at 25 oc? 6. consider the following reaction co2 (g) h2 (g) co (g) h2o (g) calculate the value of the equilibrium constant, kc, for the above system, if.

How Long It Takes Trash To Decompose Infographic The tendency of a substance. to decompose or to react with other substances, to undergo a transition from one type of state to another, to redistribute in space. can be expressed by one and the same quantity—its chemical potential μ. the strength of this tendency, meaning the numerical value of μ, is not unchangeable but. Other attempts to decompose ammonia by eliminating the effect of temperature was carried out in 1928 by mclennan and greenwood, who investigated the ammonia decomposition in a cathode ray tube. in their study, it was initially determined that the electric discharge generated in the reactor completely decomposed the ammonia in a very short time, and that the chamber composed of glass had an. Distillation under reduced pressure. distillation under reduced pressure is used to purify a liquid that has a tendency to decompose when heated to a high temperature. under the conditions of reduced pressure, the liquid will boil at a temperature lower than its boiling point, and as a result, the liquids will not degrade as they would otherwise. Chemistry questions and answers. consider the decomposition of nitrous oxide, laughing gas, 2 n2o (g) = 2 n2 (g) o2 (g) at 25⁰c, kc is 7.3 x 1034 (a) based on the information given, what can you say about the rate of decomposition of the reaction? (b) based on the information given, does nitrous oxide have a tendency to decompose into.
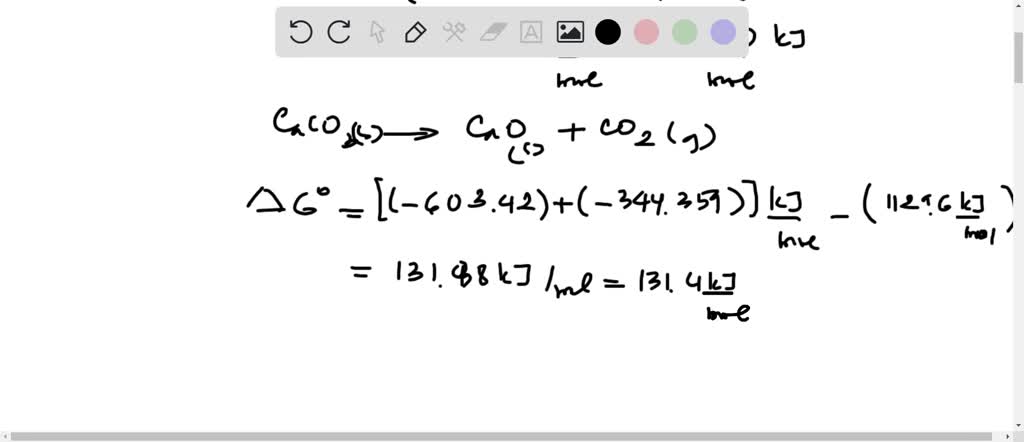
Solved Using Data In Appendix L Calculate δr G Values For The Distillation under reduced pressure. distillation under reduced pressure is used to purify a liquid that has a tendency to decompose when heated to a high temperature. under the conditions of reduced pressure, the liquid will boil at a temperature lower than its boiling point, and as a result, the liquids will not degrade as they would otherwise. Chemistry questions and answers. consider the decomposition of nitrous oxide, laughing gas, 2 n2o (g) = 2 n2 (g) o2 (g) at 25⁰c, kc is 7.3 x 1034 (a) based on the information given, what can you say about the rate of decomposition of the reaction? (b) based on the information given, does nitrous oxide have a tendency to decompose into. Description. lt = trenddecomp(a) finds trends in a vector of data using singular spectrum analysis (ssa), which assumes an additive decomposition of the data such that a = lt st r. in this decomposition, lt is the long term trend in the data, st is the seasonal, or oscillatory, trend (or trends), and r is the remainder. However, it has a tendency to decompose into water ($\mathrm{h} {2} \mathrm{o}(l)$) and oxygen ($\mathrm{o} {2}(g)$). step 3 4 step 3: the reason for this is that the $\delta h {f}^{\circ}$ of water is more negative than that of hydrogen peroxide.
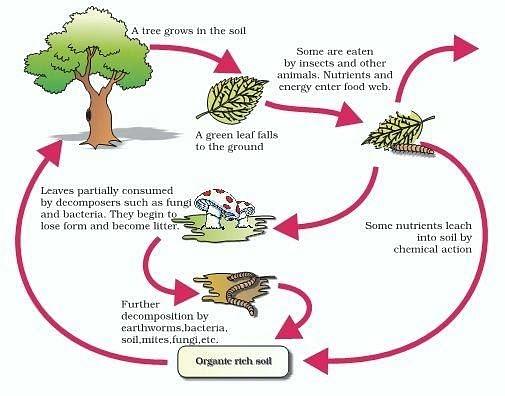
Decomposition Types Stages Process Factors Description. lt = trenddecomp(a) finds trends in a vector of data using singular spectrum analysis (ssa), which assumes an additive decomposition of the data such that a = lt st r. in this decomposition, lt is the long term trend in the data, st is the seasonal, or oscillatory, trend (or trends), and r is the remainder. However, it has a tendency to decompose into water ($\mathrm{h} {2} \mathrm{o}(l)$) and oxygen ($\mathrm{o} {2}(g)$). step 3 4 step 3: the reason for this is that the $\delta h {f}^{\circ}$ of water is more negative than that of hydrogen peroxide.

Comments are closed.