The Ph Scale Explained
The Ph Scale Of Acid And Bases Psiberg The ph scale measures whether there is more hydronium or hydroxide in a solution. in other words, it tells us how basic or acidic the solution is. a lower ph means something is more acidic, also known as a stronger acid. a higher ph means it is more alkaline or a stronger base. chemistry classes will often use a litmus test to identify acids. The ph scale shows how acidic a substance is. it can be measured using a ph meter which gives a numerical value. the ph scale ranges from 0 (very. acidic. close. acidic a word which describes.

The Ph Scale Of Common Chemicals Range and indication. the range of ph goes from 0 to 14. a value less than 7 indicates that water is acidic. a value greater than 7 indicates that water is alkaline. a value equal to 7 shows that water is neutral. each whole ph value below 7 is ten times more acidic than the next higher value. for example, a ph of 5 is ten times more acidic than 6. The ph range is commonly given as zero to 14, but a ph value can be less than 0 for very concentrated strong acids or greater than 14 for very concentrated strong bases. [2] the ph scale is traceable to a set of standard solutions whose ph is established by international agreement. [3]. The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. for example, a ph of 3 is ten times more acidic than a ph of 4. likewise, a ph of 3 is one hundred times more acidic than a ph of 5. similarly a ph of 11 is ten times more basic than a ph of 10. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. the term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ion —which ordinarily ranges between about 1 and 10 −14 gram equivalents per litre—into numbers between 0 and 14.
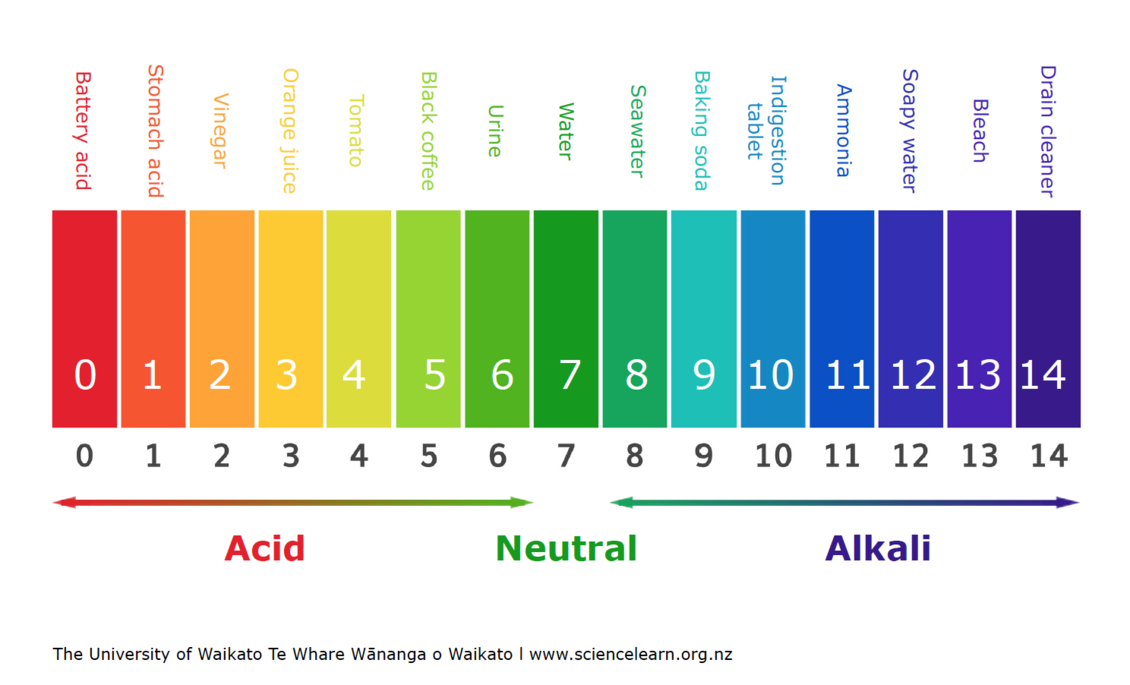
Ph Scale вђ Science Learning Hub The ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. for example, a ph of 3 is ten times more acidic than a ph of 4. likewise, a ph of 3 is one hundred times more acidic than a ph of 5. similarly a ph of 11 is ten times more basic than a ph of 10. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. the term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ion —which ordinarily ranges between about 1 and 10 −14 gram equivalents per litre—into numbers between 0 and 14. The ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. To be more precise, ph is the negative logarithm of the hydrogen ion concentration: ph = −log [h ] the square brackets around the h automatically mean "concentration" to a chemist. what the equation means is just what we said before: for each 1 unit change in ph, the hydrogen ion concentration changes ten fold.
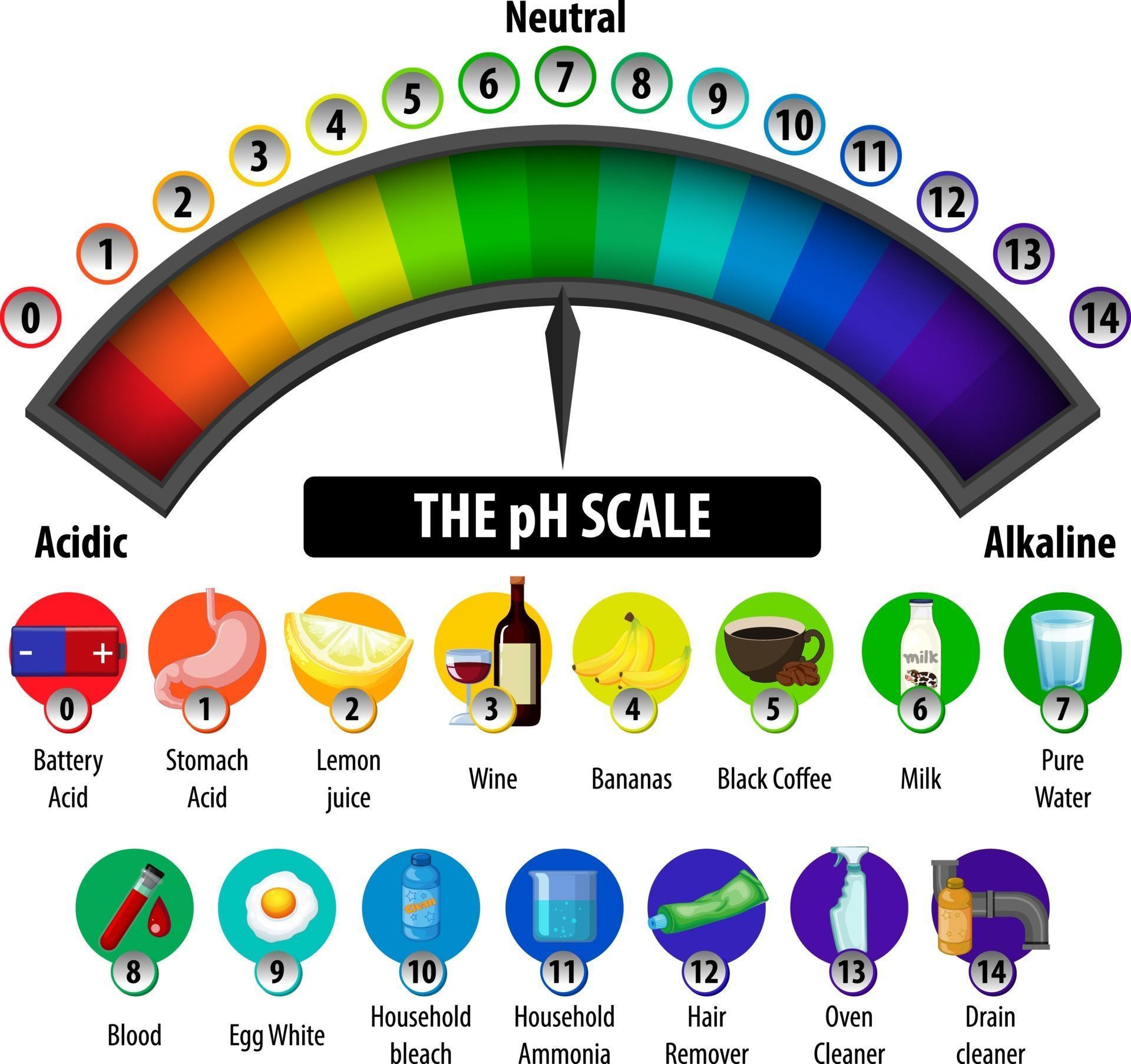
The Ph Scale Diagram On White Background 2988621 Vector Art At Vecteezy The ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. chemicals with ph values from 0 up to 7 are acids, those with a ph value of 7 are neutral, and those with ph values greater than 7 up to 14 are bases. To be more precise, ph is the negative logarithm of the hydrogen ion concentration: ph = −log [h ] the square brackets around the h automatically mean "concentration" to a chemist. what the equation means is just what we said before: for each 1 unit change in ph, the hydrogen ion concentration changes ten fold.
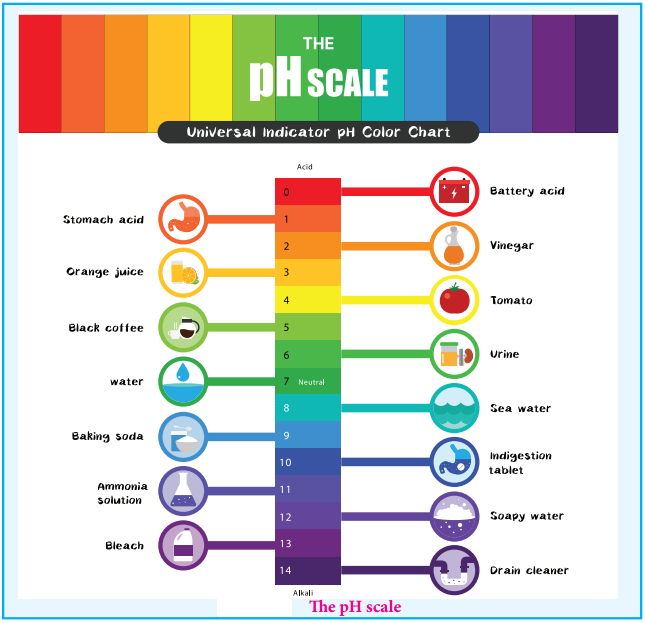
The Ph Scale

Comments are closed.