Titrations Of Polyprotic Acids
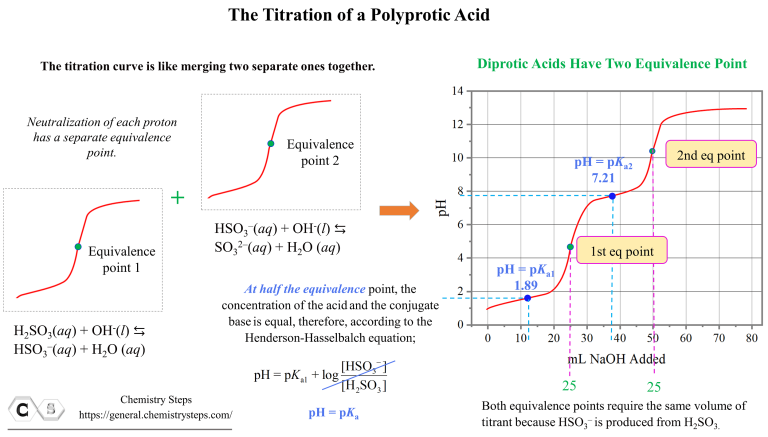
Titration Of A Polyprotic Acids Chemistry Steps The weak polyprotic acid (analyte) is in green and is titrated with teh strong base (the titrant) in red. (cc by; heather yee via libretexts) when an acid is titrated, there is an equivalence, or stoichiometric, point, which is when the moles of the strong base added equal of the moles of weak acid present. however, when a weak polyprotic acid. D30.2 titration of polyprotic acids and bases. when a polyprotic acid is titrated, there are usually multiple equivalence points. for example, when h 2 so 3 is titrated with naoh, there are two equivalence points corresponding to the two acidic protons from the h 2 so 3 molecule. there are also as many midpoints as there are equivalence points.
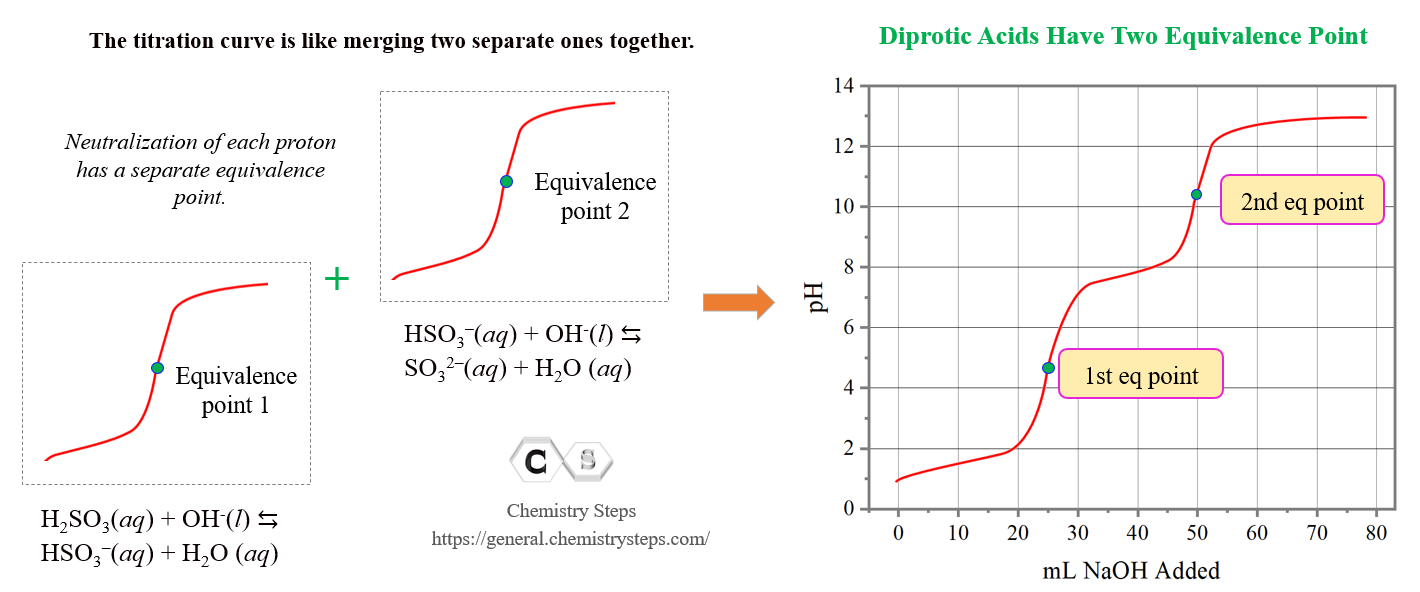
Titration Of A Polyprotic Acids Chemistry Steps A typical acid base titration curve can be obtained, for example, based on the reaction of hcl and naoh: check the original article for more details and explanations, and once the concepts are clear, let’s discuss the titration of a polyprotic acid. for example, h 2 so 3 is a diprotic acid, and its dissociation can be divides in two parts. Titrating a polyprotic acid with a strong base produces a ph curve with as many equivalence points as there are acidic protons on the acid. the pkₐ values fo. Introduction. polyprotic acids are specific acids that are capable of losing more than a single proton per molecule in acid base reactions. (in other words, acids that have more than one ionizable h atom per molecule). protons are lost through several stages (one at each stage), with the first proton being the fastest and most easily lost. Polyprotic acids. polyprotic acids are acids that can lose several protons per molecule. they can be further categorized into diprotic acids and triprotic acids, those which can donate two and three protons, respectively. the best way to demonstrate polyprotic acids and bases is with a titration curve.

Comments are closed.