Unit 11 Lesson 11 Polyprotic Titration Ph Curves

Unit 11 Lesson 11 Polyprotic Titration Ph Curves Youtube Identifying pka values and major chemical species along a polyprotic acid ph titration curve. How does ph relate to pk a at any point on the titration curve? out of all the acid dissociation constants for the dissociation of the protons for a weak polyprotic acid, which is the largest? given that \( k {a1}=5.9 \times 10^{ 3} \) and \( k {a2}=6.0 \times 10^{ 6} \), calculate the ph after titrating 70 ml of 0.10 m h 2 so 3 with 50 ml of 0.

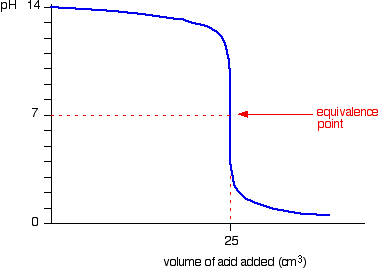
Ph Titration Curves Chemistry Libretexts Figure 17.4.3: the titration of (a) a weak acid with a strong base and (b) a weak base with a strong acid. (a) as 0.200 m naoh is slowly added to 50.0 ml of 0.100 m acetic acid, the ph increases slowly at first, then increases rapidly as the equivalence point is approached, and then again increases more slowly. Because hpo 4 2− is a very weak acid, pk a,3 has a high ph value, and the third step cannot be resolved using 0.100 m naoh as the titrant. figure: titration curve for phosphoric acid. the curve for the titration of 25.0 ml of 0.100 m h 3 po 4 solution with 0.100 m naoh. species in solution at each midpoint are shown. note the two distinct. Titration curve. the curve of a polyprotic acid will have multiple peaks, meaning multiple equilibrium points. at 1 2 each equilibrium point, ph = pka. ph meter. use to measure ph, do not leave out of solution when on. procedure. titrate the unknown acid with naoh by adding naoh in increments to the unknown. measure ph after each addition. If you calculate the values, the ph falls all the way from 11.3 when you have added 24.9 cm 3 to 2.7 when you have added 25.1 cm 3. note: if you need to know how to calculate ph changes during a titration, you may be interested in my chemistry calculations book .
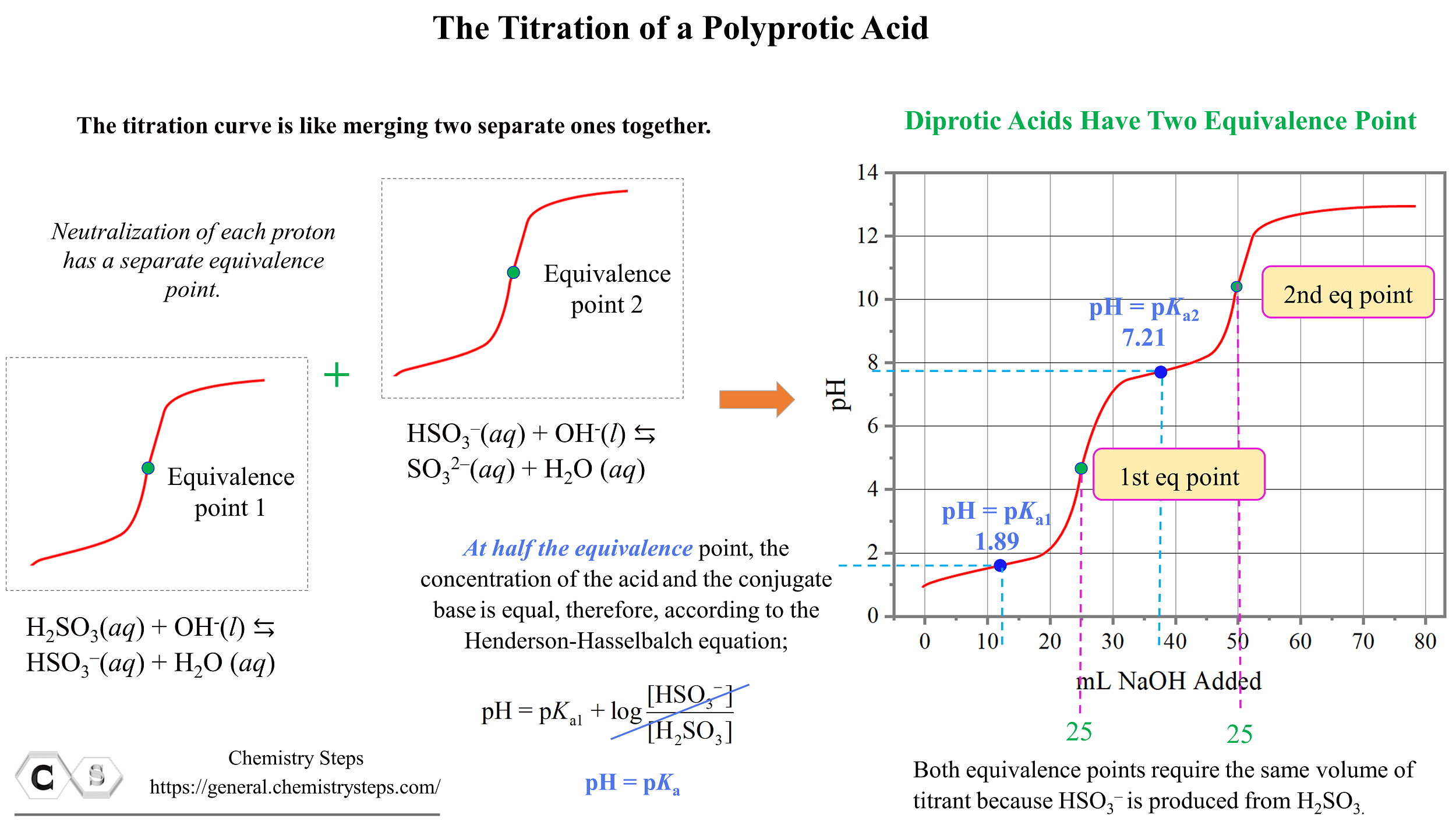
Titration Of A Polyprotic Acids Chemistry Steps Titration curve. the curve of a polyprotic acid will have multiple peaks, meaning multiple equilibrium points. at 1 2 each equilibrium point, ph = pka. ph meter. use to measure ph, do not leave out of solution when on. procedure. titrate the unknown acid with naoh by adding naoh in increments to the unknown. measure ph after each addition. If you calculate the values, the ph falls all the way from 11.3 when you have added 24.9 cm 3 to 2.7 when you have added 25.1 cm 3. note: if you need to know how to calculate ph changes during a titration, you may be interested in my chemistry calculations book . Hso 3– (aq) h 2 o (l) ⇆ so 32 (aq) h 3 o (aq) ka2 = 6.4 x 10 8. when titrated with a base, one of the protons reacts with the hydroxide first to form the conjugate base hso 3–, which then reacts with the base forming the so 32 conjugate base. let’s say we are titrating 25.0 ml of 0.100 m h2so3 with 0.100 m naoh. Cha x cha x. x x 0 h a 0 x x. and the calculations can be as simple as. [h ] = ca. or. [h ] = (kaca)0.5 but life isn’t that easy. some acids and bases are more fortunate than others in that they are given multiple reactive sites at birth. these molecules are known as polyprotic acids and bases and include:.
Ph Titration Curves Chemistry Libretexts Hso 3– (aq) h 2 o (l) ⇆ so 32 (aq) h 3 o (aq) ka2 = 6.4 x 10 8. when titrated with a base, one of the protons reacts with the hydroxide first to form the conjugate base hso 3–, which then reacts with the base forming the so 32 conjugate base. let’s say we are titrating 25.0 ml of 0.100 m h2so3 with 0.100 m naoh. Cha x cha x. x x 0 h a 0 x x. and the calculations can be as simple as. [h ] = ca. or. [h ] = (kaca)0.5 but life isn’t that easy. some acids and bases are more fortunate than others in that they are given multiple reactive sites at birth. these molecules are known as polyprotic acids and bases and include:.

Chapter 11 Polyprotic Titration Curve Calcs 1 Chm 214 111 Youtube

Comments are closed.