Validation Of A Method For The Quantification Of Influenza
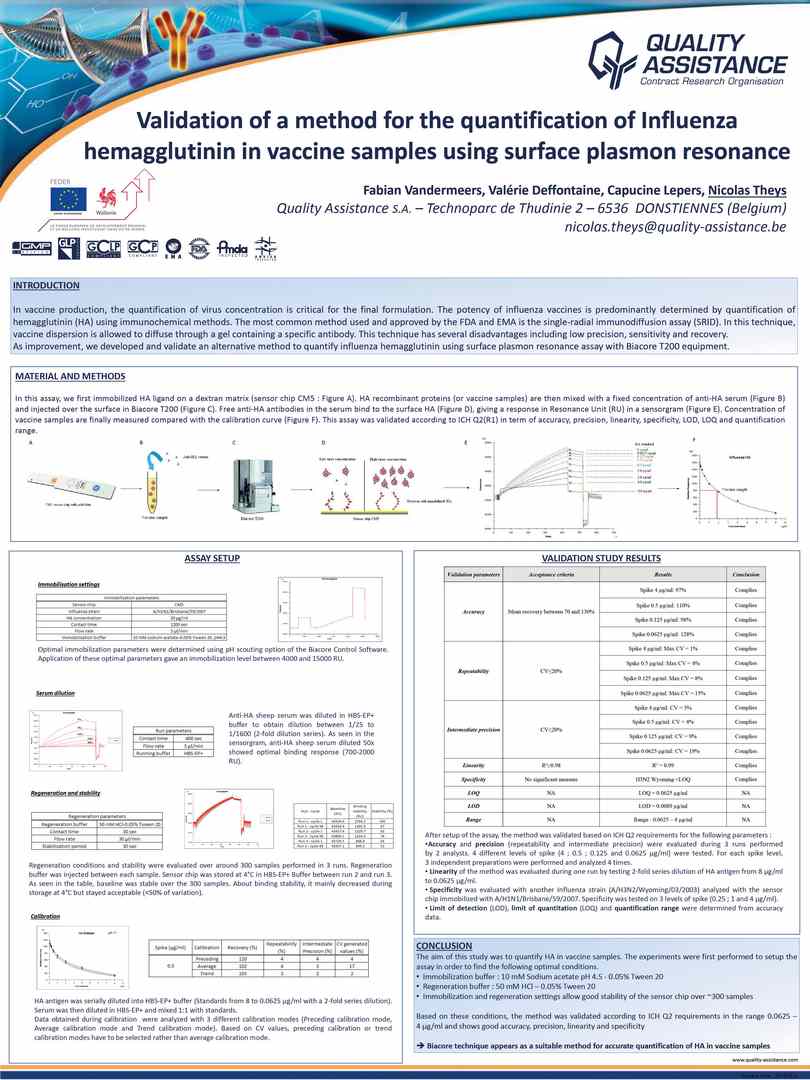
Validation Of A Method For The Quantification Of Influenza The most frequently utilized quantification method is the titration by plaque assay or 50% tissue culture infectious dose estimation by tcid 50. however, both methods are time consuming. moreover, some iav strains form hardly visible plaques, and the evaluation of tcid 50 is subjective. employment of immuno staining into the classic protocols. Thus, this study aimed to develop and standardize a universal absolute quantification for influenza a by reverse transcription quantitative pcr (rt qpcr), using a plasmid dna. the assay showed efficiency (eff%) 98.6, determination coefficient (r 2 ) 0.998, linear range 10^ 1 to 10^ 10 , limit of detection (lod) 6.77, limit of quantification (loq) 20.52 copies reaction.
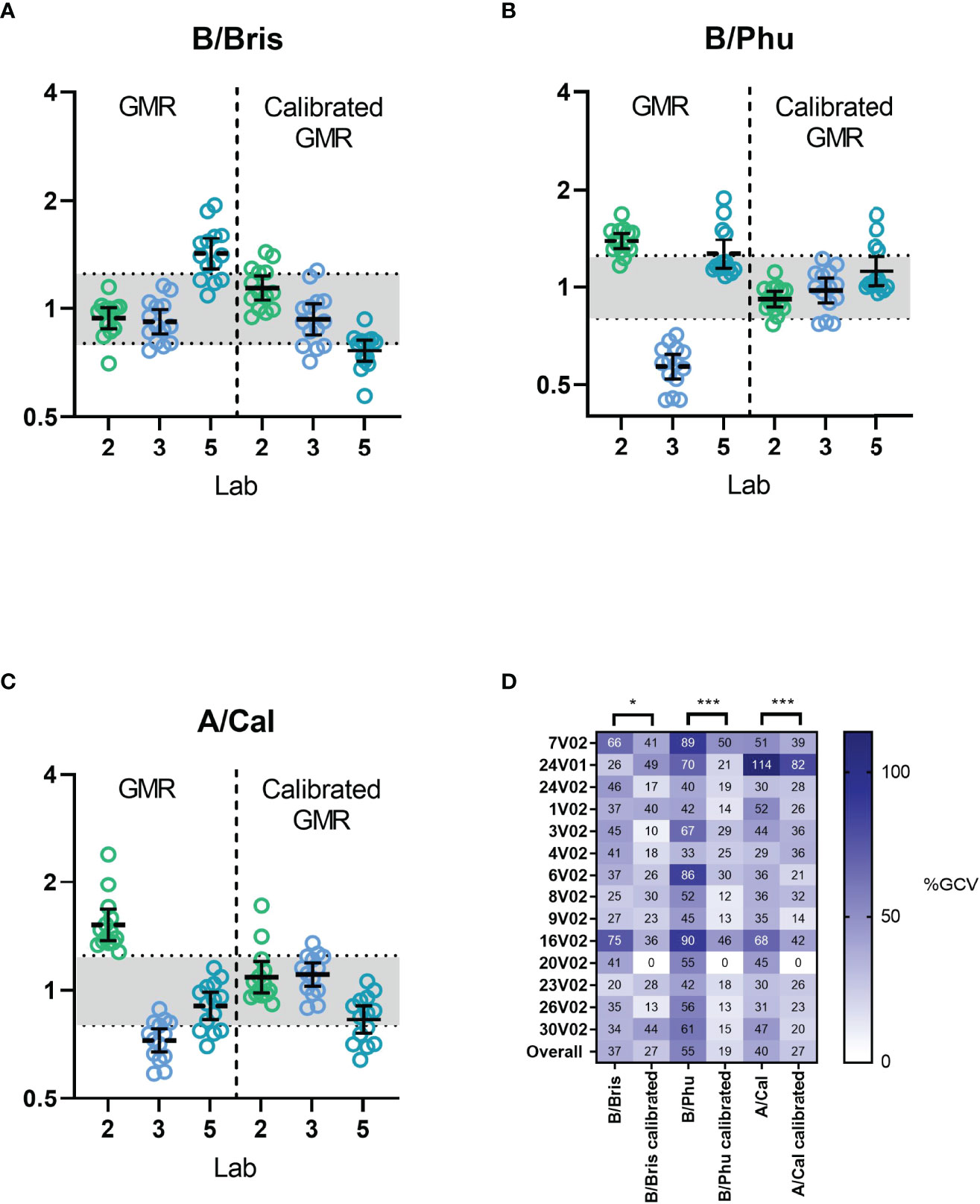
Frontiers Validation Of A Harmonized Enzyme Linked Lectin Assay Ella The developed method was validated according to the us fda guidelines for bioanalytical method validation [24,25]. selectivity was evaluated at a lower limit of quantitation (lloq) at a concentration of 3 µg ml (10% of c max ), where the sample of lloq was analyzed, peak area was noted and compared with the response obtained for the blank plasma sample at the retention time of fvir. This is due, as discussed in a previous review of influenza vlp quantification methods , to the particularity of each quantification method and their intrinsic bias. it should be emphasized that the hemagglutination or srid assays, routinely used to quantify vlps in past studies, do not have the ability to make a distinction between real virus like particles and impurities [ 19 , 22 ]. Therefore, rt pcr detection methods were preferentially used as the quantification method of determining the equivalent infectious viral load in environmental waters. the adsorption of viral particles to a membrane is due to electrostatic interactions but depend on both the environmental characteristics and surface properties of the virus ( 20 ). The world health organization called for a global pandemic influenza vaccine action plan including the development of new technologies. a rapid and reliable method for the quantification of influenza total particles is crucially needed to support the development, improvement and validation of novel influenza vaccine manufacturing platforms.

Using Experimental Human Influenza Infections To Validate A Viral Therefore, rt pcr detection methods were preferentially used as the quantification method of determining the equivalent infectious viral load in environmental waters. the adsorption of viral particles to a membrane is due to electrostatic interactions but depend on both the environmental characteristics and surface properties of the virus ( 20 ). The world health organization called for a global pandemic influenza vaccine action plan including the development of new technologies. a rapid and reliable method for the quantification of influenza total particles is crucially needed to support the development, improvement and validation of novel influenza vaccine manufacturing platforms. The assurance of vaccine potency is important for the timely release and distribution of influenza vaccines. as an alternative to single radial immunodiffusion (srid), we report a new quantitative. Efficiency of fish in free virus particles. (a) schematic depiction and summary of the standard fish based virus detection protocol with incubation times for the individual steps 35.(b.

Development And Validation Of A Mass Spectrometry Based Analytical The assurance of vaccine potency is important for the timely release and distribution of influenza vaccines. as an alternative to single radial immunodiffusion (srid), we report a new quantitative. Efficiency of fish in free virus particles. (a) schematic depiction and summary of the standard fish based virus detection protocol with incubation times for the individual steps 35.(b.

Comments are closed.