What Is A 3d Phase Diagram Example
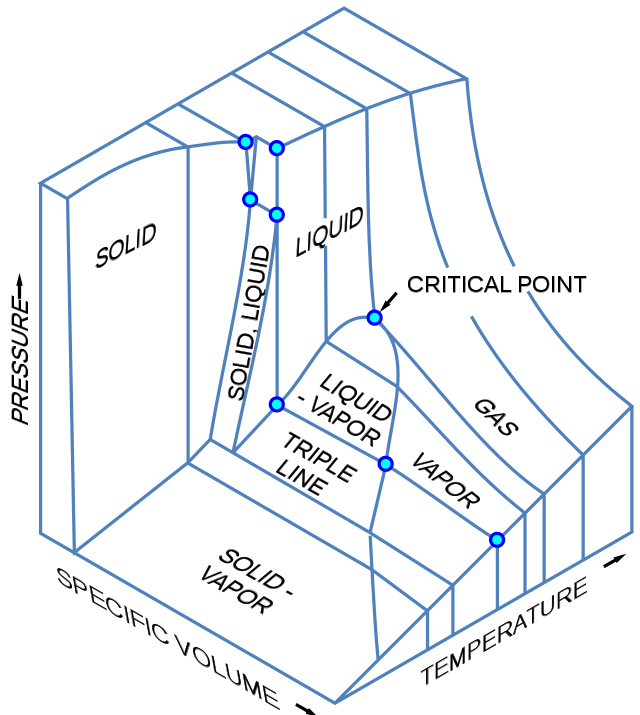
Phase Diagrams Chemtalk A 3d phase diagram is a type of graph in which three different conditions (such as p, v, t) are plotted along the cartesian axes. > it shows the conditions at which different phases occur and coexist at equilibrium. the equilibrium conditions are shown as curved surfaces in 3d, with areas for solid, liquid, and vapour phases and areas where two or three phases can coexist in equilibrium. for. Three dimensional phase change diagrams plot three thermodynamic variables and show regions of space corresponding to different phases. in this type of diagram, we have a triple line instead of a triple point, and coexistence surfaces instead of coexistence curves. below is a generic 3d diagram plotting temperature, pressure, and specific volume.
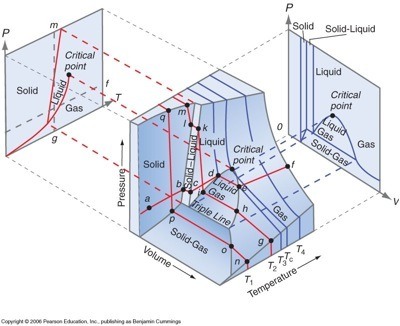
What Is A 3d Phase Diagram Example One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. Phase diagram. a phase diagram represents the various physical states or phases of matter at different pressures and temperatures. in other words, it summarizes the effect of pressure and temperature on the nature of a substance. a phase diagram is divided into three areas representing the substances’ solid, liquid, and gaseous phases [1 4]. Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in.

What Is A 3d Phase Diagram Example Vrogue Co Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in. The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. the curves on the phase diagram show the points where the free energy (and other derived properties) becomes non analytic: their derivatives with respect to the coordinates (temperature and. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.
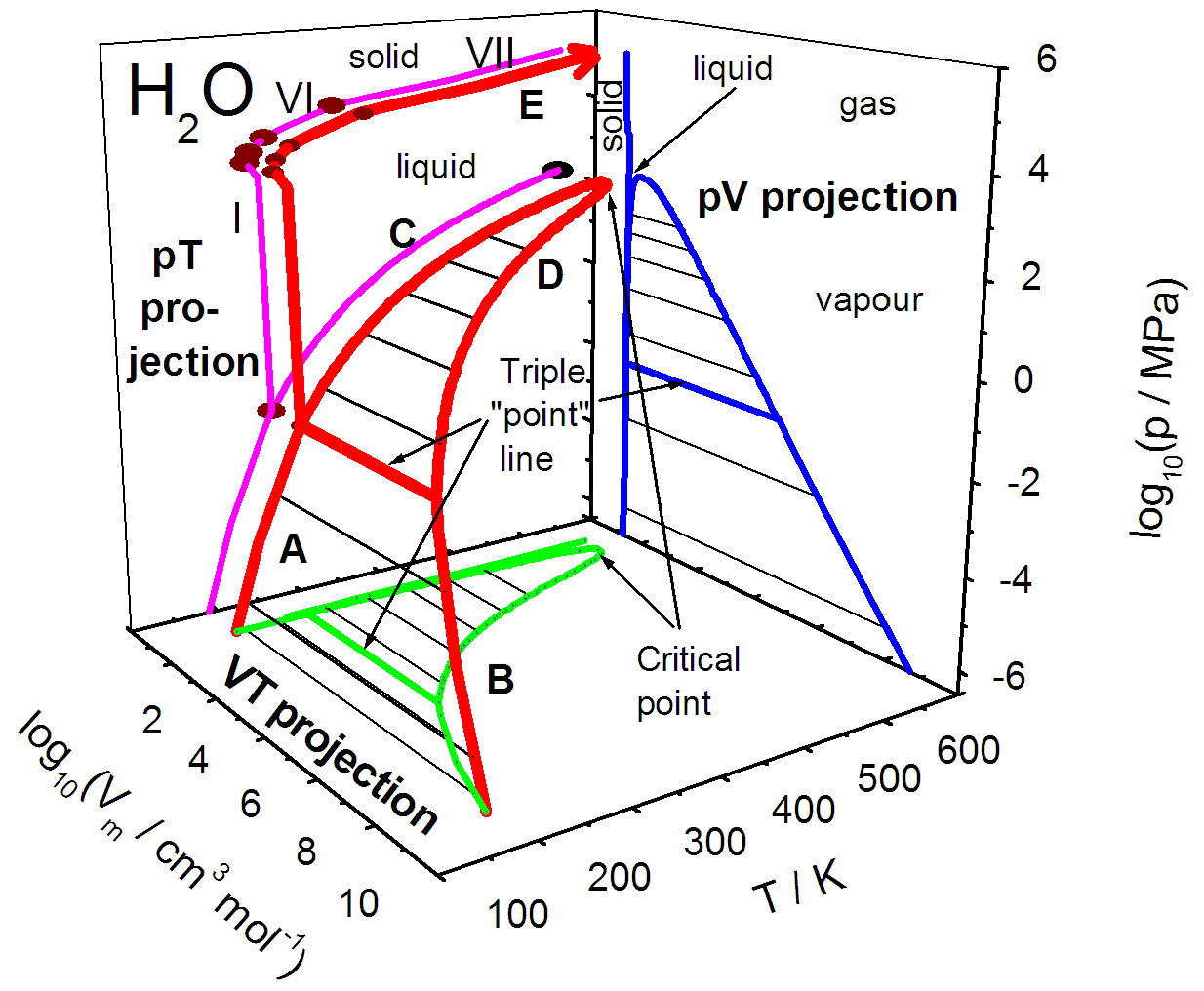
Phase Diagram Using Matlab At Evan Ward Blog The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas. the curves on the phase diagram show the points where the free energy (and other derived properties) becomes non analytic: their derivatives with respect to the coordinates (temperature and. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.

Phase Diagrams Chemtalk

Comments are closed.