What Is A Base In Chemistry Acids And Bases
Acids And Bases In chemistry, bases react with acids. they release hydroxide ions, donate electrons, or accept protons. in chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. learn about the properties of bases and see examples of bases and their uses. The sodium hydroxide, calcium carbonate and potassium oxide are examples of bases. a base is a substance that reacts with hydrogen ions and can neutralize the acid. most bases are minerals which form water and salts by reacting with acids. bases include the metal oxides, hydroxides, and carbonates. q8.
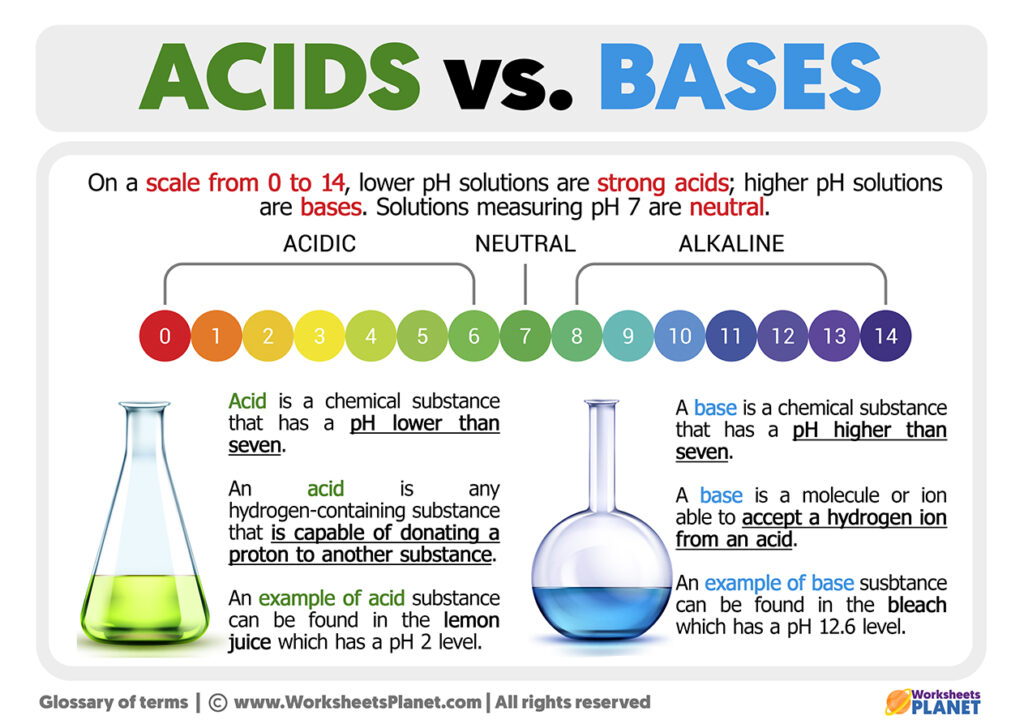
Acid And Bases Differences The earliest definition of acids and bases is arrhenius's definition which states that: an acid is a substance that forms hydrogen ions h when dissolved in water, and; a base is a substance that forms hydroxide ions oh when dissolved in water. for example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{ }}\) when it. Bases also change the color of indicators. litmus turns blue in the presence of a base, while phenolphthalein turns pink. bases do not react with metals in the way that acids do. bases react with acids to produce a salt and water. figure \(\pageindex{1}\): phenolphthalein indicator in presence of base. Arrhenius definition. "an acidic substance is one whose molecular unit contains at least one hydrogen atom that can dissociate, or ionize, when dissolved in water, producing a hydrated hydrogen ion and an anion." hydrochloric acid. hcl → h (aq) cl –(aq) sulfuric acid. h 2 so 4 → h (aq) hso 4–(aq). Acid base chemistry involves accepting or donating either protons or electron pairs. acid base chemistry is a fundamental aspect of chemical science that plays a crucial role in our daily lives. its applications range from industrial processes to biological systems. understanding acid base chemistry is not just essential for scientists, but.
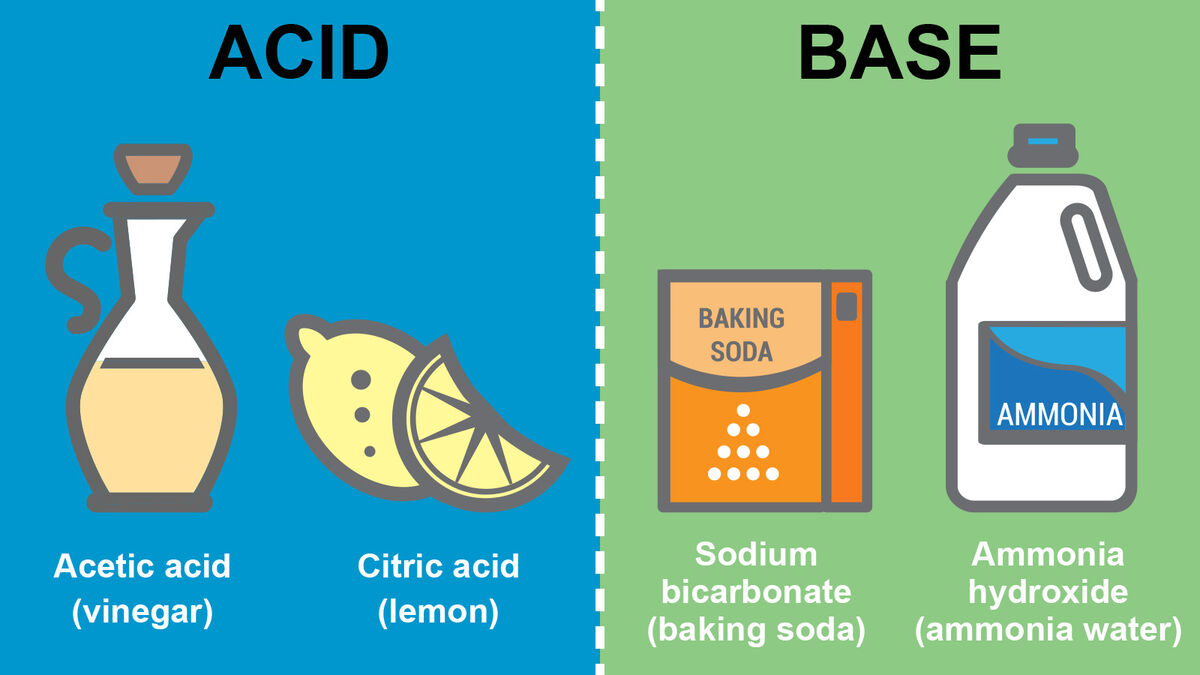
How To Identify Bases And Acids Arrhenius definition. "an acidic substance is one whose molecular unit contains at least one hydrogen atom that can dissociate, or ionize, when dissolved in water, producing a hydrated hydrogen ion and an anion." hydrochloric acid. hcl → h (aq) cl –(aq) sulfuric acid. h 2 so 4 → h (aq) hso 4–(aq). Acid base chemistry involves accepting or donating either protons or electron pairs. acid base chemistry is a fundamental aspect of chemical science that plays a crucial role in our daily lives. its applications range from industrial processes to biological systems. understanding acid base chemistry is not just essential for scientists, but. The chemical difference between acids and bases is that acids produce hydrogen ions and bases accept hydrogen ions. a base is a substance that neutralises acids. when bases are added to water, they split to form hydroxide ions, written as oh . we call a base that has been added to water an alkaline solution. To identify acids from bases, and the relative strength of each, chemists tend to use a ph scale. seven is neutral. anything with a ph below 7 is acidic. anything with a ph above 7 is basic. one of the earliest tests to determine acids from bases was the litmus test. a chemical patch turned red for acids, blue for bases.

A Level Chemistry Revision Physical Chemistry Acids And Bases The chemical difference between acids and bases is that acids produce hydrogen ions and bases accept hydrogen ions. a base is a substance that neutralises acids. when bases are added to water, they split to form hydroxide ions, written as oh . we call a base that has been added to water an alkaline solution. To identify acids from bases, and the relative strength of each, chemists tend to use a ph scale. seven is neutral. anything with a ph below 7 is acidic. anything with a ph above 7 is basic. one of the earliest tests to determine acids from bases was the litmus test. a chemical patch turned red for acids, blue for bases.

Comments are closed.