What Is Atomic Spectroscopy
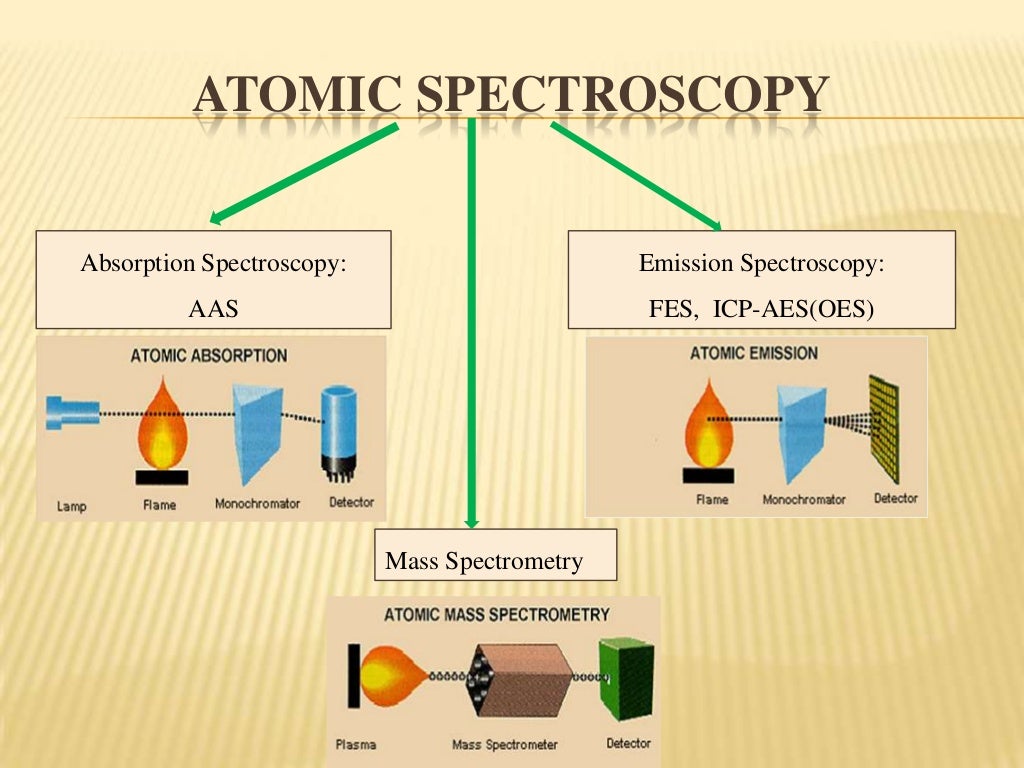
Atomic Spectroscopy Basic Principles And Instruments Atomic spectroscopy. in physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. it can be divided by atomization source or by the type of spectroscopy used. Atomic spectroscopy is the study of the electromagnetic radiation absorbed or emitted by atoms. there are three types of atomic spectroscopy and they are: atomic emission spectroscopy: this involves the transfer of energy from the ground state to an excited state.
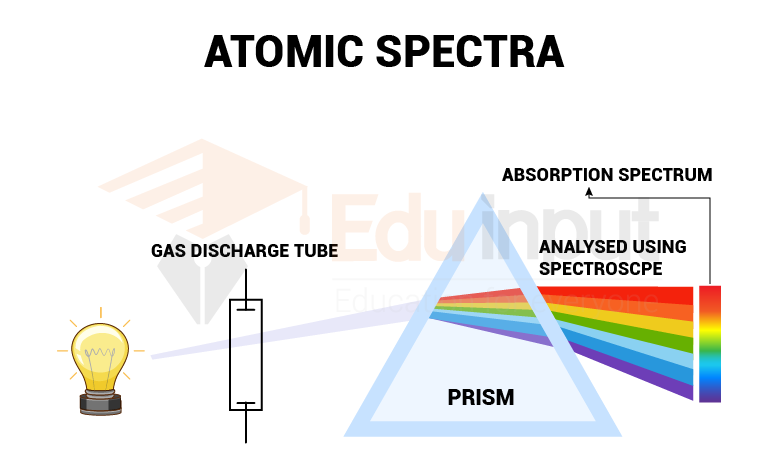
юааatomicюаб юааspectraюаб юааatomicюаб юааspectroscopyюаб Rydbergтащs Formula Atomic spectroscopy. atomic spectroscopy (including atomic absorption spectrometry, atomic emission spectrometry, and atomic fluorescence spectrometry) is of use across the span of reactive adhesive technologies. for example, the cure of anaerobic adhesives on nonreactive surfaces is usually assisted by the use of an active metal based primer. Atomic spectroscopy is the determination of elemental composition by its electromagnetic or mass spectrum. the study of the electromagnetic spectrum of elements is called optical atomic spectroscopy. electrons exist in energy levels within an atom. Therefore, performing atomic spectroscopy on most samples involves the utilization of an atomization source, which is a device that has the ability to convert molecules to atoms. it is also important to recognize that the absorption or emission spectrum of a neutral atom will be different than that of its ions (e.g., cr 0 , cr 3 , cr 6 all. This page titled 4.2: understanding atomic spectra is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by elizabeth gordon. the ground state of an atom is the lowest energy state of the atom. when those atoms are given energy, the electrons absorb the energy and move to a higher energy level.

Introduction To Atomic Spectroscopy Youtube Therefore, performing atomic spectroscopy on most samples involves the utilization of an atomization source, which is a device that has the ability to convert molecules to atoms. it is also important to recognize that the absorption or emission spectrum of a neutral atom will be different than that of its ions (e.g., cr 0 , cr 3 , cr 6 all. This page titled 4.2: understanding atomic spectra is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by elizabeth gordon. the ground state of an atom is the lowest energy state of the atom. when those atoms are given energy, the electrons absorb the energy and move to a higher energy level. E = mc2 (1.4.1) (1.4.1) e = m c 2. both atomic emission and atomic absorption spectroscopy can be used to analyze samples. atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. this light is typically in the visible or. The photon energy due to an electron transition between an upper atomic level k(of energy ek) and a lower level iis. 2 of 31 3 3 1999 8:09 am atomic spectroscopy: an introduction. e= ek ei= h= hc= hc vac, (1) where is the frequency, the wavenumber in vacuum, and vacthe wavelength in vacuum.
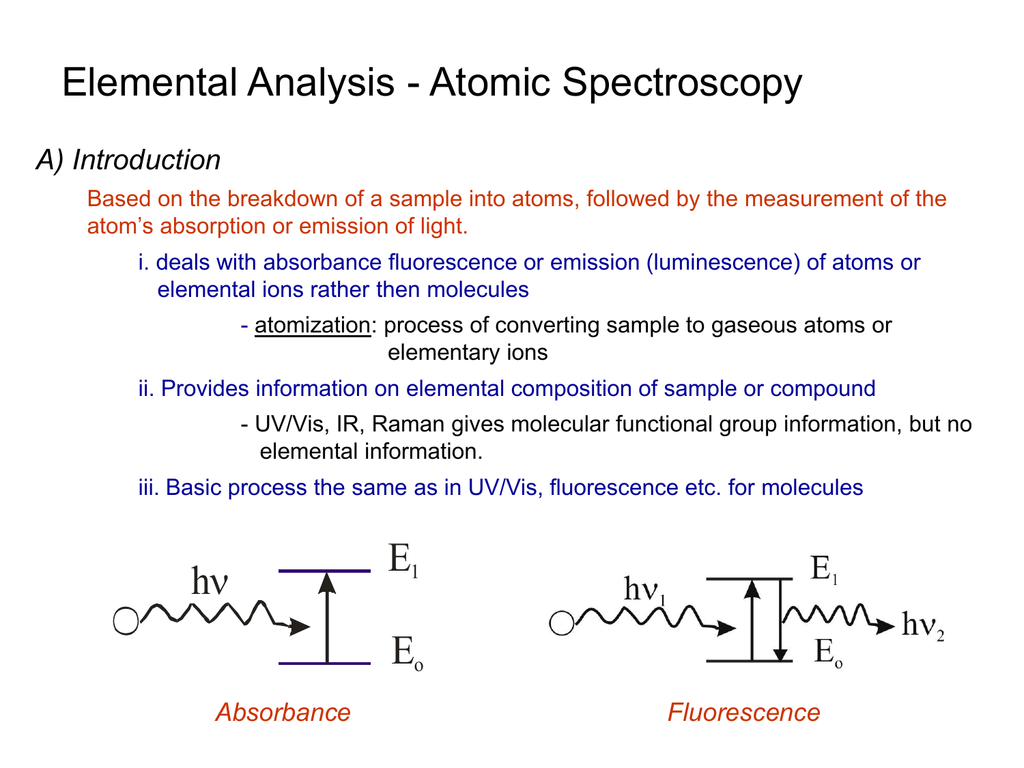
Chapters 8 10 Atomic Spectroscopy E = mc2 (1.4.1) (1.4.1) e = m c 2. both atomic emission and atomic absorption spectroscopy can be used to analyze samples. atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed by atomic absorption. this light is typically in the visible or. The photon energy due to an electron transition between an upper atomic level k(of energy ek) and a lower level iis. 2 of 31 3 3 1999 8:09 am atomic spectroscopy: an introduction. e= ek ei= h= hc= hc vac, (1) where is the frequency, the wavenumber in vacuum, and vacthe wavelength in vacuum.

Atomic Spectroscopy Testing Posts

Comments are closed.