What Is The Original Color Of Bromothymol Blue Btb

What Is The Original Color Of Bromothymol Blue Btb Researchgate Bromothymol blue (also known as bromothymol sulfone phthalein and btb) is a ph indicator. it is mostly used in applications that require measuring substances that would have a relatively neutral ph (near 7). a common use is for measuring the presence of carbonic acid in a liquid. it is typically sold in solid form as the sodium salt of the acid. Syed raju ali. king abdulaziz university. btb is a weak acid and it is almost red in color. however, when it reacts with acidic solution then it turns into yellow color and blue color after.

Bromothymol Blue Color Change Chart A Visual Reference Of Charts Description. bromothymol blue is a member of the class of 2,1 benzoxathioles that is 2,1 benzoxathiole 1,1 dioxide in which both of the hydrogens at position 3 have been substituted by 3 bromo 4 hydroxy 5 isopropyl 2 methylphenyl groups. it has a role as an acid base indicator, a dye and a two colour indicator. Bromothymol blue turns yellow in solutions with ph < 6.5, green between 6.5 7.2 and blue at ph > 7.2. bromothymol blue slowly oxidizes in air, turning lilac purple, though this does not affect its ph sensitivity. physical. bromothymol blue is an odorless colorless solid, that turns lilac purple over long periods of time in contact with air. 0.1 = 0.0001x. 0.1 0.0001 = x. thus, x is equal to 1000. bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. to prepare a solution that is used as a ph indicator, we should dissolve 0.10 g in an 8.0 cm3 n 50 naoh and then dilute it with water to 250 cm3. Bromothymol blue is an indicator in the ph range from 6.0 to 7.6. bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. it contains a ph indicator , bromothymol blue, which causes the medium to change from yellow to blue green; it contains antimicrobial agents, chloramphenicol.

Bromothymol Blue Color Chart 0.1 = 0.0001x. 0.1 0.0001 = x. thus, x is equal to 1000. bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. to prepare a solution that is used as a ph indicator, we should dissolve 0.10 g in an 8.0 cm3 n 50 naoh and then dilute it with water to 250 cm3. Bromothymol blue is an indicator in the ph range from 6.0 to 7.6. bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. it contains a ph indicator , bromothymol blue, which causes the medium to change from yellow to blue green; it contains antimicrobial agents, chloramphenicol. The green ionized form is the active form of the indicator, and it changes to the yellow unionized form in the presence of co2 or when the ph of a solution changes. the color change of bromothymol blue is due to the movement of protons (h ) between the two forms of the indicator. when the ph of a solution increases, the concentration of h ions. Definition. bromothymol blue is a ph indicator known for its color change from yellow to blue as the solution changes from acidic to basic. congrats on reading the definition of bromothymol blue. now let's actually learn it. ok, let's learn stuff.
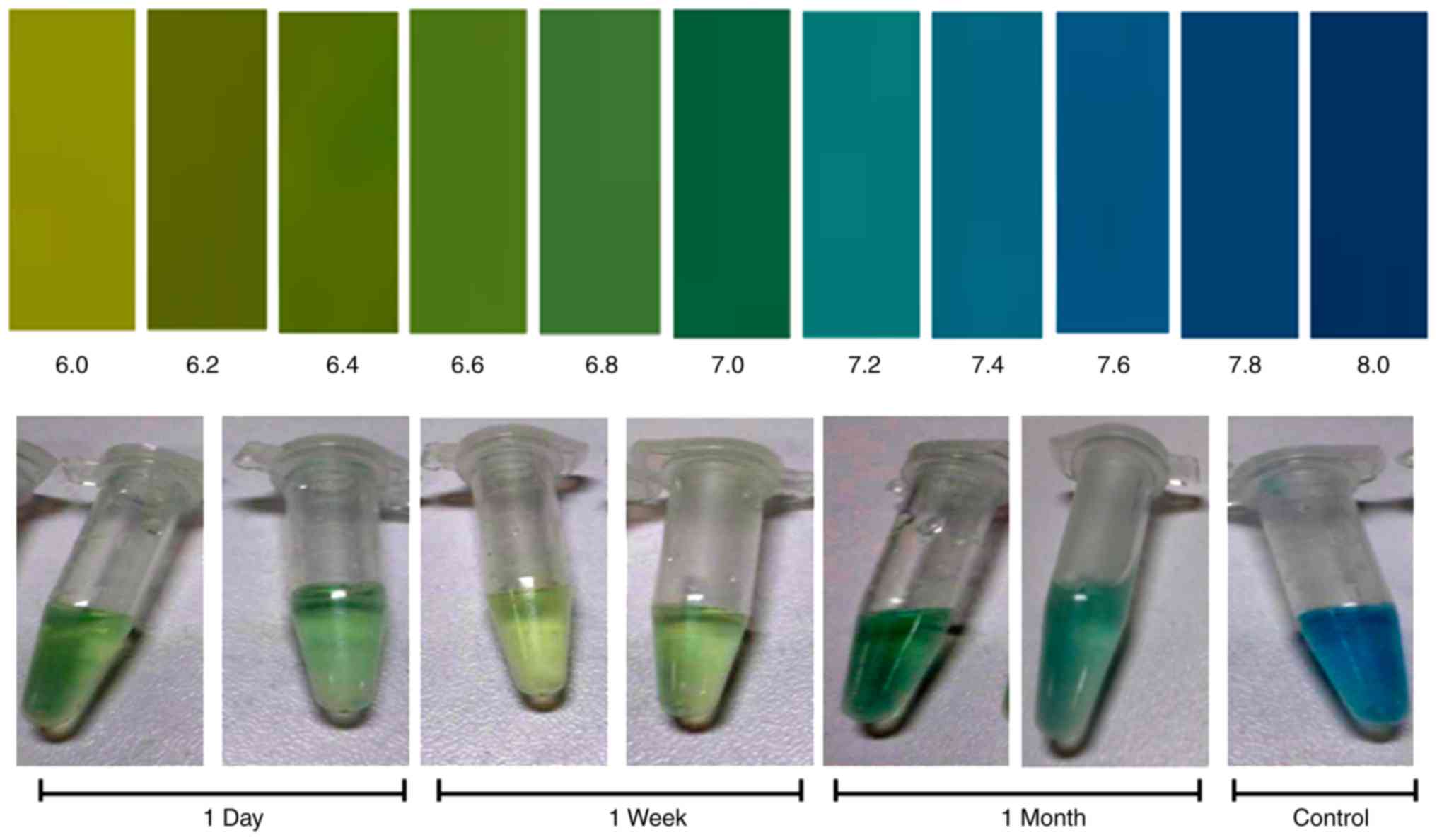
Bromothymol Blue Color Chart A Visual Reference Of Charts Chart Master The green ionized form is the active form of the indicator, and it changes to the yellow unionized form in the presence of co2 or when the ph of a solution changes. the color change of bromothymol blue is due to the movement of protons (h ) between the two forms of the indicator. when the ph of a solution increases, the concentration of h ions. Definition. bromothymol blue is a ph indicator known for its color change from yellow to blue as the solution changes from acidic to basic. congrats on reading the definition of bromothymol blue. now let's actually learn it. ok, let's learn stuff.

Bromothymol Blue Definition Uses Formula Study

Comments are closed.